Abstract
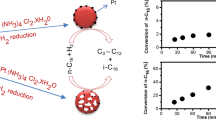
Pore-widths and pore-size distributions of 0.5 wt% Pt-CsxH3-xPW12O40 have been studied by means of adsorption of various molecules. For the distributions of micropore and mesopore, isotherms of Ar and N2 adsorption were analyzed, respectively. Pt-Cs2.1H0.9PW12O40 possessed only ultramicropores. On the other hand, the pores of Pt-CsxH3-xPW12O40 (x = 2.3, 2.5, 2.8 and 3.0) showed bimodal distributions in the range from micropore to mesopore, and the widths of both pores tended to increase as the Cs content increased. From the amounts and rates of adsorption for n-butane and isobutane, the pore width of Pt-Cs2.1H0.9PW12O40 was determined to be close to the molecular size of n-butane, that is, 0.43 nm. The fraction of external surface area in the total surface area of Pt-Cs2.1H0.9PW12O40 was estimated to be only 0.06 from the adsorption of 1,3,5-trimethylbenzene and t-plot of N2 adsorption. Pt- Cs2.1H0.9PW12O40 exhibited a shape selectivity due to the uniform ultramicropores and small external surface area; it catalyzed the oxidation of n-butane but not that of isobutane. SEM and TEM measurements revealed the primary crystallites and their aggregated states.
Similar content being viewed by others
References
P.B. Venute, Micropor. Mater. 2 (1994) 297.
S.M. Csicsery, Pure Appl. Chem. 58 (1986) 841; Zeolites 4 (1984) 202.
K. Tanabe and W.G. Holderich, Appl. Catal. 181 (1999) 399.
M.T. Pope, Heteropoly and Isopoly Oxometallates, (Springer-Verlag, 1983).
T. Okuhara, N. Mizuno and M. Misono, Advan. Catal. 41 (1996) 113.
K. Sano, H. Uchida and S. Wakabayashi, Catal. Surv. Jpn 3 (1999) 55.
T. Okuhara, T. Nishimura and M. Misono, Chem.Lett. (1995) 155.
Y. Yoshinaga, K. Seki, T. Nakato and T. Okuhara, Angew. Chem. Int. Ed. Engl. 36 (1997) 2833.
Y. Yoshinaga and T. Okuhara, J. Chem. Soc. Faraday Trans. 94 (1998) 2235.
T. Okuhara, T. Yamada, K. Seki, K. Johkan and T. Nakato, Micropor. Mesopor. Mater. 21 (1998) 637.
S.T. Gregg, M.M. Tayyab, J. Chem. Soc. Faraday Trans. 1, 74 (1978) 348.
T. Okuhara, H. Watanabe, T. Nishimura, K. Inumaru and M. Misono, Chem. Mater. 12 (2000) 2230.
T. Okuhara and T. Nakato, Catal. Surv. Jpn 2 (1999) 31.
T. Okuhara, T. Nishimura and M. Misono, in: Proc. 11th Intern. Congr. Catal., eds. J.W. Hightower, W.N. Delgass, E. Iglesia and A.T. Bell, (Elsevier, Amsterdam, 1996), p. 581.
T. Ito, K. Inumaru and M. Misono, J. Phys. Chem. B. 191 (1997) 9958.
K. Inumaru, T. Ito and M. Misono, Micropor. Mesopor. Mater. 21 (1998) 629.
A. Kaliadima, L.A. Perez-Maqueda and E. Matijivic, Langmuir 13 (1997) 3733.
L.A. Perez-Maqueda and E. Matijevic, Chem. Mater. 10 (1998) 1430.
A. Saito and H.C. Foley, Micropor. Mater. 3 (1995) 531.
D. Dollimore and G.R. Heal, J. Appl. Chem. 14 (1964) 109.
T. Yamada, K. Johkan and T. Okuhara, Micropor. Mesopor. Mater. 26 (1998) 109.
S.J. Gregg and W.K.S. Sing, Adsorption, Surface Area and Porosity, 2nd ed, (Academic Press, London, 1982).
D.W. Breck, Zeolite Molecular Sieves, (Wiley, New York, 1974).
D.W. Breck, W.G Eversol, R.M. Milton, T.B. Reed and T.L. Thomas, J. Am. Chem. Soc. 78 (1956) 5963.
D.E.W. Vaughan and R.J. Lussier, Proc. 5th Intern. Conf. Zeolites, 1980, p. 94.
A.L. McClellan and H.F.J. Harnsberger, Colloid Interface Sci. 23 (1967) 577.
J. Rouquerol, D. Avnir, C.W. Fairbridge, D.H. Everett, J.H. Haynes, N. Pernicone, J.D.F. Ramsay, K.S. Sing and K.K. Unger, Pure Appl. Chem. 66 (1994) 1739.
T. Yamada, Y. Yoshinaga and T. Okuhara, Bull. Chem. Soc. Jpn. 71 (1998) 2727.
N. Mizuno and M. Misono, Chem. Lett. (1987) 961.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshinaga, Y., Suzuki, T., Yoshimune, M. et al. Pore Structure and Shape Selectivity of Platinum-Promoted Cesium Salts of 12-Tungstophosphoric Acid. Topics in Catalysis 19, 179–185 (2002). https://doi.org/10.1023/A:1015203923067
Issue Date:
DOI: https://doi.org/10.1023/A:1015203923067