Abstract
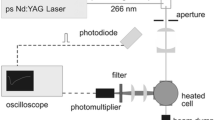
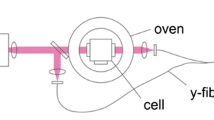
The fluorescence quenching of pyrene (PY) by carbon tetrabromide (CBr4) at pressures of up to 400 MPa in n-hexane was investigated. It was found that the fluorescence quenching is not fully, but nearly, diffusion-controlled. From the pressure-induced solvent viscosity dependence, the quenching rate constant, k q , was separated into the contributions of the bimolecular rate constant in the solvent cage, k bim, and that for diffusion, k diff. Using the values of k diff separated, together with those of the diffusion coefficient of CBr4, the diffusion coefficients of PY were successfully estimated. This analysis was applied to the quenching systems of 9,10-dimethylanthracene (DMEA)/CBr4 and of PY/O2 and DMEA/O2 that were studied previously. Using the values of k diff for these systems, together with those of the corresponding diffusion coefficients of the fluorophore or quencher, the diffusion coefficients of DMEA and O2 were also evaluated. Based on the results, the pressure-induced solvent viscosity, η, dependence on the diffusion coefficients is discussed.
Similar content being viewed by others
REFERENCES
J. B. Birks, Photophysics of Aromatic Molecules (Wiley–Interscience, New York, 1970), p. 518.
J. Saltiel and B. W. Atwater, Advances in Photochemistry (Wiley–Interscience, New York, 1987), Vol. 14, p. 1.
J. B. Birks, Organic Molecular Photophysics (Wiley, New York, 1973), p. 403.
S. A. Rice, in Comprehensive Chemical Kinetics. Diffusion-Limited Reactions, C. H. Bamford, C. F. H. Tripper, and R. G. Compton, eds. (Elsevier, Amsterdam, 1985), Vol. 25.
W. R. Ware, J. Phys. Chem. 66:455 (1962).
W. R. Ware and J. S. Novros, J. Phys. Chem. 70:3246 (1966).
T. M. Nemzek and W. R. Ware, J. Chem. Phys. 62:477 (1975).
H. Yasuda, A. D. Scully, S. Hirayama, M. Okamoto, and F. Tanaka, J. Am. Chem. Soc. 112:6847 (1990).
S. Hirayama, H. Yasuda, A. D. Scully, and M. Okamoto, J. Phys. Chem. 98:4609 (1994).
M. Okamoto, F. Tanaka, and S. Hirayama, J. Phys. Chem. A 102:10703 (1998).
M. Okamoto, J. Phys. Chem. A 104:5029 (2000).
M. Okamoto, J. Phys. Chem. A 104:7518 (2000).
M. Okamoto, O. Wada, F. Tanaka, and S. Hirayama, J. Phys. Chem. A 105:566 (2001).
M. Okamoto and O. Wada, J. Photochem. Photobiol. A Chem. 138:87 (2001).
M. Okamoto and F. Tanaka, submitted for publication.
J. H. Dymond and L. A. Woolf, J. Chem. Soc. Faraday Trans. I 78:991 (1982).
M. Okamoto and H. Teranishi, J. Phys. Chem. 88:5644 (1984).
P. W. Bridgman, Proc. Am. Acad. Arts Sci. 61:57 (1926).
D. W. Brazier and G. R. Freeman, Can. J. Chem. 47:893 (1969).
C. M. B. P. Oliveira and W. A. Wakeham, Int. J. Thermophys. 13:773 (1992).
M. Okamoto and S. Sasaki, J. Phys. Chem. 95:6548 (1991).
The radial distribution function, g(rMgQ), at the closest approach distance, rMgQ(=rMg+rQ), the sum of the radius of the hard-sphere solutes in a hard-sphere solvent with radius, rS, is given by [23] g(rMgQ)= 11-y +3y(1-y)2 Rrred rS S+ 2y2 (1-y)3 Rrred rS S2 (A1) where rred=rMgrQ/rMgQ, and y is the packing fraction, given in terms of the molar volume of the solvent, VS, by y= 4NApr3 S 3VS (A2) By using the values of rS, rQ, and rMg estimated by the method of Bondi [24], together with the data on the solvent density [18–20], g(rMgQ) was calculated by Eq. (A1).
Y. Yoshimura and M. Nakahara, J. Chem. Phys. 81:4080 (1984).
A. Bondi, J. Phys. Chem. 68:441 (1964).
D. F. Evans, T. Tominaga, and C. Chan, J. Solut. Chem. 8:461(1979).
D. F. Evans, T. Tominaga, and H. T. Davis, J. Chem. Phys. 74:1298 (1981).
A. Spernol and K. Wirtz, Z. Naturforsch. 8A:522 (1953).
A. Gierer and K. Wirtz, Z. Naturforsch. 8A:532 (1953).
The values of Fsw i (full) and fSW i (trunc) were evaluated by Eq. (10) and by neglecting the second parenthetical quantity in Eq. (10), respectively [2].
A. Schumpe and P. Lühring, J. Chem. Eng. Data 35:24 (1990).
E. Sada, S. Kito, T. Oda, and Y. Ito, Chem. Eng. J. 10:155 (1975).
A. Akgerman and J. L. Gainer, Ind. Eng. Fundam. 11:373 (1972).
B. A. Kowert and N. C. Dang, J. Phys. Chem. A 103:779 (1999).
J. Jonas, Acc. Chem. Res. 17:74 (1984).
J. Jonas, D. Hasha, and S. G. Huang, J. Phys. Chem. 84:109 (1980).
J. Jonas, D. Hasha, and S. G. Huang, J. Chem. Phys. 71:3996 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Okamoto, M. Diffusion Coefficients Estimated by Dynamic Fluorescence Quenching at High Pressure: Pyrene, 9,10-Dimethylanthracene, and Oxygen in n-Hexane. International Journal of Thermophysics 23, 421–435 (2002). https://doi.org/10.1023/A:1015157419045
Issue Date:
DOI: https://doi.org/10.1023/A:1015157419045