Abstract
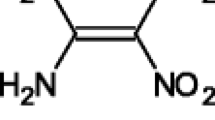
Purpose. To characterize the electrochemical behavior of the photodegradation product of nifedipine, i.e., 2,6-dimethyl-4-(2-nitrosophenyl)-3,5-pyridine-carboxylic acid dimethyl ester (NPD) in different electrolytic media. We also evaluated the interaction between free radicals generated from NPD and xeno/endobiotics.
Methods. Tast polarography, differential pulse polarography, and cyclic voltammetry were used for the characterization. Controlled potential electrolysis and ultraviolet-visible spectroscopy were used to generate and to detect the nitroso radical anion.
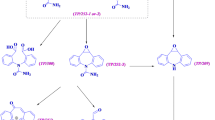
Results. In protic media, the NPD derivative gave a reversible well-defined peak either on Hg or glassy carbon electrodes in a reaction involving two electrons and two protons to give the hydroxylamine derivative. In mixed aqueous-organic media (pH 9) and in aprotic media, nitroso radical anion was isolated and characterized, exhibiting second-order dimerization rate constant (k2) values of 11,300 ± 210 [Ms]−1 and 8,820 ± 78 [Ms]−1, respectively. Reactivity of the nitroso radical anion with relevant pharmacologic targets revealed a significant interaction with the tested endo/xenobiotics (cysteamine, GSH, N-acetylcysteine, and adenine).
Conclusions. Both in mixed and aprotic media, NPD generated free-radical species, the nitroso radical anion. Taking into account their respective interaction rate constants, the following tentative rank order of reactivity can be established as follows: cysteamine > N-acetylcysteine > GSH > adenine.
Similar content being viewed by others
REFERENCES
P. D. Henry. Comparative pharmacology of calcium antagonists: nifedipine, verapamil and diltiazem. Am. J. Cardiol. 46:1047–1058 (1980).
P. Jakobsen, O. Lederballe Pedersen, and E. Mikkelsen. Gas chromatographic determination of nifedipine and one of its metabolites using electron capture detection. J. Chromatogr. 162:81–87 (1979).
B. K. Logan and K. S. Patrick. Photodegradation of nifedipine relative to nitrendipine evaluated by liquid and gas chromatography. J. Chromatogr. 529:175–181 (1990).
Y. Matsuda, R. Teraoka, and I. Sugimoto. Comparative evaluation of photostability of solid-state nifedipine under ordinary and intensive light irradiation conditions. Int. J. Pharmaceutics 54: 211–221 (1989).
M. Morad, Y. E. Goldman, and D. R. Trentham. Rapid photochemical inactivation of Ca2+-antagonists shows that Ca2+ entry directly activates contraction in frog heart. Nature 304:635–638 (1983).
R. Teraaka. Y. Matsuda and Sugimoto. Quantitative design for photostabilization of nifedipine by using titanium dioxide and/or tartrazine as colorants in model film coating systems. J. Pharm. Pharmacol 41:293–297 (1988).
N. K. Gibbs. N. J. Traynor, B. E. Johnson, and J. Ferguson. In vitro photostability of nifedipine: sequential induction of toxic and non-toxic photoproducts with UV-A radiation. J. Photochem. Photobiol. B. 13:275–288 (1992).
N. Hayase. Y-I. Itagaki., S. Ogawa., S. Akutsu, and S-I. Y. Abiko. Newly discovered photodegradation products of nifedipine in hospital prescriptions. J. Pharm. Sci. 83:532–538 (1994).
R. S. Nicholson. Semiempirical procedure for measuring with stationary electrode polarography rates of chemical reactions involving the product of electron transfer. Anal. Chem 38:1406 (1966).
G. Bontempelli, F. Magno, G. Mozzochin, and R. Seeger. Linear sweep and cyclic voltammetry. Annali di Chimica. 79:138–147 (1989).
M. L. Olmstead and R. G. Hamilton. and R.S. Nicholson Voltammetry Theory for the Disproportionation Reaction and Spherical Diffusion. Anal. Chem 41:260–266 (1969).
M. L. Olmstead, R. G. Hamilton, and R. S. Nicholson. Theory of cyclic voltammetry for a dimerization reaction initiated electrochemically. Anal. Chem. 41:260–266 (1969).
L. J. NÚñez-Vergara, F. García, M. M. Domínguez, J. De la Fuente, and J. A. Squella. In situ reactivity of the electrochemically generated nitro radical anion from nitrendipine with glutathione, adenine and uracil. J. Electroanal. Chem. 381:215–219 (1995).
L. J. NÚñez-Vergara. J. C. Sturm, A. Alvarez-Lueje, C. Olea-Azar, C. Sunkel, and J.A. Squella electrochemical oxidation of 4-methyl-1,4-dijydropyridines in protic and aprotic media. J. Electrochem. Soc. 146:1478–1485 (1999).
A. G. Gonzalez, F. Pablos, and A. Asuero. Corrections Factors for the glass electrode revisited. Talanta 39:91 (1992).
A. G. Asuero and M. A. Herrador, and A.G. González,, Estimation of pH and autoproteolysis constants in mixtures of aliphatic amides with water: medium effect on the 4-aminobenzene system. Talanta 40:479–484 (1993).
E. Laviron, A. Vallat, and R. Meunier-Prest, The reduction mechanism of aromatic nitro compounds in aqueous medium Part V. The reduction of nitrosobenzene between pH 0.4 and 13. J. Electroanal. Chem. 379:427–435 (1994).
J. H. Tocher. R. C. Knigth, and D. I. Edwards. Electrochemical characteristics of nitro-heterocyclic compounds of biologic interest. II. Nitrosochoramphenicol. Free Rad. Res. Commun. 5:319–326 (1989).
A. Streitwiesser and C. H. Heatcock. In: Química Orgánica, Interamericana, (1996).
P. Zuman and S. Bhavdeep. Addition, reduction, and oxidation reactions of nitrosobenzene. Chem Rev. 94:1621–1641 (1994).
B. Kastering. P. L. Meites, I. M. Kolthoff. In Zuman (ed.), Progress in Polarography, vol 3, John Wiley & Sons, New York, 1972 pp. 259–266.
M. R. Asirvatham and D. Hawley. Electron-transfer processes: the electrochemical and chemical behavior of nitrosobenzene. J. Electroanal. Chem. 57:179–190 (1974).
L. J. NÚñez-Vergara, M. E. Ortiz, S. Bollo, and J. A. Squella. Electrochemical generation and reactivity of free radical redox intermediates from ortho-and meta-nitro substituted 1,4-dihydropyridines. Chem. Biol. Interact. 106:1–14 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Núñez-Vergara, L.J., Bollo, S., Fuentealba, J. et al. Electrochemical and Spectroelectrochemical Behavior of the Main Photodegradation Product of Nifedipine: The Nitrosopyridine Derivative. Pharm Res 19, 522–529 (2002). https://doi.org/10.1023/A:1015112216360
Issue Date:
DOI: https://doi.org/10.1023/A:1015112216360