Abstract
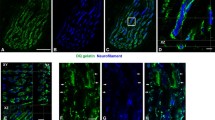
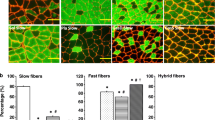
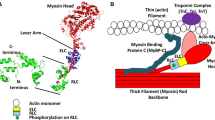
Four different phenotypes of slow muscle fibers, characterized by differential epitope expression in the slow/β myosin heavy chain (MyHC) isoform, have been identified in adult rabbit masseter muscle. We investigated the role of post-translational phosphorylation in the expression of these four phenotypes. Serial cryostat sections were treated either with alkaline phosphatase to dephosphorylate proteins in the tissue, or with a brain kinase solution and ATP to phosphorylate them, and then stained, using four antibodies that bind specifically to the slow/β MyHC isoform. In sections pre-treated with phosphatase, immunoreactivity to antibody A4.840 was abolished, but it could be restored by subsequent kinase/ATP treatment or ATP alone, indicating that the expression of its epitope requires phosphorylation. Phosphatase treatment resulted in an exposure of the epitope for antibody A4.951 in cells that normally bind this antibody only weakly or not at all, but since heat treatment alone produced similar effects, the role of phosphorylation in this enhancement is less certain. Immunoreactivity to antibodies S58 and BA-D5 were not influenced by phosphatase pre-treatment. Kinase/ATP treatment was only effective in changing antibody binding when tissues already had been phosphatase treated. We interpret these results to mean that sites of potential phosphorylation may already be occupied by O-linked glycosylation.
Similar content being viewed by others
References
Alberts B, Bray D, Lewis J, Ra. M, Roberts K and Watson JD (1994). Molecular Biology of the Cell. (1294 pp.) Garland, New York.
Bredman JJ, Wessels A, Weijs WA, Korfage JA, Soffers CA and Moorman AF (1991) Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochem J 23: 160–170.
Butler-Browne G and Whalen R (1984) Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol 102: 324–449.
Crow M and Stockdale F (1986) Myosin expression and specialization among the earliest muscle fibers of the developing avian limb. Dev Biol 113: 238–254.
English AW, Eason J, Pol M, Schwartz G and Shirley A (1998) Different phenotypes among slow/β myosin heavy chain-containing fibers of rabbit masseter muscle: a novel type of diversity in adult muscle. J Muscle Res Cell Motil 19: 525–535.
Favre B, Turowski P and Hemmings BA (1997) Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem 272: 13, 856-13, 863.
Harris AJ, Fitzsimons RB and McEwan JC (1989) Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development 107: 751–769.
Hart GW (1997) Dynamic O-linked glycosylation of nuclear and cytoskeletal proteins. Ann Rev Biochem 66: 315–335.
Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA and Blau HM (1993) Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol 158: 183–199.
Kavinsky CJ, Umeda PK, Levin JE, Sinha AM, Nigro JM, Jakovcic S and Rabinowitz M (1984) Analysis of cloned mRNA sequences encoding subfragment 2 and part of subfragment 1 of α-and β-myosin heavy chains of rabbit heart. J Biol Chem 259: 2775–2781.
Korfage JA and Van Eijden TM (1999) Regional differences in fibre type composition in the human temporalis muscle. J Anat 194: 355–362.
Korfage JA and Van Eijden TM (2000) Myosin isoform composition of the human medial and lateral pterygoid muscles. J Dent Res 79: 1618–1625.
Lefaucheur L, Hoffman R, Okamura C, Gerrard D, Léger J, Rubinstein N and Kelly A (1997) Transitory expression of a cardiac myosin heavy chain in a subpopulation of secondary generation muscle fibers in the pig. Dev Dyn 210: 106–116.
Maggs AM, Taylor-Harris P, Peckham M and Hughes SM (2000) Evidence for differential post-translational modifications of slow myosin heavy chain during murine skeletal muscle development. J Muscle Res Cell Motil 21: 101–113.
Mandell JW and Banker G (1996) A spatial gradient of τ protein phosphorylation in nascent axons. J Neurosci 16: 5727–5740.
Monemi M, Eriksson PO, Kadi F, Butler-Browne GS and Thornell LE (1999) Opposite changes in myosin heavy chain composition of human masseter and biceps brachii muscles during aging. J Muscle Res Cell Motil 20: 351–361.
Murakami N, Kumon A, Matsumura S, Hara S and Ikenaka T (1988) Phosphorylation of the heavy chain of skeletal muscle myosin by casein kinase II: localization of the phosphorylation site to the amino terminus. J Biochem 103: 209–211.
Rowlerson A, Pope B, Murray J, Whalen RB and Weeds AG (1981) A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil 2: 415–438.
Schiaffino S and Reggiani C (1996) Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev 76: 371–423.
Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K and Lomo T (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10: 197–205.
Shelton GD, Cardinet GH 3rd and Bandman E. (1988) Expression of fiber type specific proteins during ontogeny of canine temporalis muscle. Muscle Nerve 11: 124–132.
Talmadge RJ and Roy RR (1993) Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340.
Trybus KM (1986) Myosin regulation and assembly. In Barany M (ed.) Biochemistry of Smooth Muscle Contraction. (pp. 37–46) Academic Press, New York.
Van den Steen P, Rudd PM, Dwek RA and Opdenakker G (1998) Concepts and principles of O-linked glycosylation. Crit Rev Biochem Mol Biol 33: 151–208.
Watters C (1978) A one-step biuret assay for protein in the presence of detergent. Ann Biochem 88: 695–698.
Wigston DJ and English AW (1992) Fiber-type proportions in mammalian soleus muscle during postnatal development. J Neurobiol 23: 61–70.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pol-Rodriguez, M.M., Schwartz, G.A. & English, A.W. Post-translational phosphorylation of the slow/β myosin heavy chain isoform in adult rabbit masseter muscle. J Muscle Res Cell Motil 22, 513–519 (2001). https://doi.org/10.1023/A:1015083616319
Issue Date:
DOI: https://doi.org/10.1023/A:1015083616319