Abstract
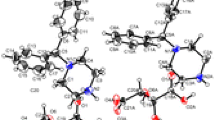
N"-Substituted isonicotinic hydrazides of the general formula Py—C(=O)—N(H)-N"=C(H)—R, where R is o- (1), m- (2), or p-nitrophenyl (3), were studied by IR spectroscopy and X-ray diffraction analysis. The position of the nitro group in these compounds has no effect on the type of the crystal structure. The crystal packings are based on stacks consisting of antiparallel planar molecules. The molecules from the adjacent stacks are linked to each other via the N—H...NPy hydrogen bonds. Depending on the position of the nitro group, the N...NPy distance increases in the series 3 > 1 > 2 and the energy of the hydrogen bonds decreases (according to the IR spectroscopic data) from 3.9 to 3.1 kcal mol–1. Analysis of the IR spectra demonstrated that the intensity of absorption in the ν(C—H) stretching region of the pyridine ring increases substantially as the the N—H...NPy hydrogen bond is strengthened. Some regularities of the changes, which are observed for the ν(NO2) bands in the spectra of the nitrophenyl-containing conjugated molecules in solutions, persist in the crystalline state.
Similar content being viewed by others
References
E. Hadjoudis, in Photochromism, Molecules and Systems, Eds. H. Durr and M. Bouas-Laurent, Elsevier, Amsterdam, 1990, 708.
Yu. P. Kitaev and B. I. Buzykin, in Gidrazony [Hydrazones], Nauka, Moscow, 1974, 381 pp. (in Russian).
Y. G. Atovmyan, L. A. Nikonova, I. I. Chuev, A. N. Utenyshev, M. Z. Aldoshina, and S. M. Aldoshin, J. Mol. Struct., 1999, 474, 167.
S. M. Aldoshin, L. A. Nikonova, E. G. Atovmyan, A. N. Utenyshev, and D. B. Frolov, Izv. Akad. Nauk, Ser. Khim., 1996, 2263 [Russ. Chem. Bull., 1996, 45, 2148 (Engl. Transl.)].
P. P. Shorygin, N. B. Lopatina, B. V. Lopatin, and T. E. Gorodilova, Izv. Akad. Nauk, Ser. Khim., 1998, 304 [Russ. Chem. Bull., 1998, 47, 297 (Engl. Transl.)].
Beilsteins Hundbuch der organischen Chemie, E III/IV, Springer, Berlin-Heidelberg-New York-London-Paris-Tokyo, 1979, 22, No. 1, 571.
I. I. Chuev, S. M. Aldoshin, E. G. Atovmyan, D. B. Frolov, and A. N. Utenyshev, Izv. Akad. Nauk, Ser. Khim., 1996, 896 [Russ. Chem. Bull., 1996, 45, 851 (Engl. Transl.)].
G. M. Sheldrik, SHELX-86, Program for the Crystal Structure Determination, University of Cambridge (England), Cambridge, 1986.
G. M. Sheldrik, SHELXL-93, Program for the Refinement of Structure, Universität Göttingen (Germany), Göttingen, 1993.
T. G. Atovmyan, S. M. Aldoshin, and L. O. Atovmyan, Mol. Cryst. Liq. Cryst., 1990, 178, 231.
E. G. Atovmyan, I. I. Chuev, L. A. Nikonova, A. N. Utenyshev, and S. M. Aldoshin, Izv. Akad. Nauk, Ser. Khim., 1998, 1369 [Russ. Chem. Bull., 1998, 47, 1331 (Engl. Transl.)].
V. S. Korobkov, Zh. Prikl. Spektrosk., 1973, 19, 1125 [J. Appl. Spectrosc., 1973, 19 (Engl. Tranl.)].
L. N. Kuleshova and P. M. Zorkii, Acta Crystallogr., Sect. B, 1981, 37, 1363.
Yu. V. Zefirov, Kristallografiya, 1997, 42, 936 [Crystallogr. Repts., 1997, 42 (Engl. Transl.)].
P. P. Shorygin and B. V. Lopatin, Zh. Fiz. Khim., 1965, 34, 2868 [J. Phys. Chem. USSR, 1965, 34 (Engl. Transl.)].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Atovmyan, E.G., Nikonova, L.A., Filipenko, O.S. et al. Influence of the position of the nitro group on the structural and spectroscopic characteristics of N"-(nitrobenzylidene)isonicotinic hydrazides in the crystalline state. Russian Chemical Bulletin 51, 99–104 (2002). https://doi.org/10.1023/A:1015065814759
Issue Date:
DOI: https://doi.org/10.1023/A:1015065814759