Abstract
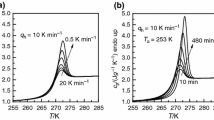
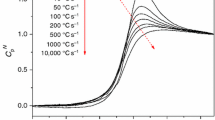
A thermodynamic study of the influence of the thermal treatments (annealing), below the glass transition temperature, on the thermal behavior and enthalpy of maltitol glass was carried out by differential scanning calorimetry (DSC). An enthalpic effect (exothermal) produced by the isothermal treatment of the quenched glass was found and measured.
The origin of the thermal effect was assigned to a physicochemical transformation of molecular associations in the solid (glass).
To achieve a correct description of the thermodynamic functions of glasses, another parameter, in addition to T and P, is introduced, namely the degree of advance of the above mentioned transformation.
Similar content being viewed by others
References
C. Bauwens-Crowet and J. C. Bauwens, Polymer, 27 (1986) 709.
M. Bahr, F. Schreir, J. Wosnitza, H. V. Lohneysen, C. Zhu, X. Qin, H. Chen and X. Wu, Europhy. Lett., 22 (1993) 443.
G. Vigier and J. Tatibouet, Polymer, 34 (1993) 4257.
A. J. Kovacs, J. M. Hutchinson and J. J. Aklonis, London: P. H. Gaskell, (1977) 153.
L. Boesch, P. B. Macedo and A. Napolitano, J. Am. Ceram. Soc., 53 (1970) 148.
J. Tauke, T. A. Litovitz and P. B. Macedo, J. Amer. Soc., 51(3) (1968) 158.
J. Perez, J. Y. Cavaille, R. Diaz Calleja, J. L. Gomez Ribelles, M. Monleon Pradas and A. Ribes Greus, Makromol. Chem., 192 (1991) 2141.
J. Perez, Polymer, 29 (1988) 483.
R. O. Davies and G. O. Jones, Proc. Roy. Soc., 217A (1953) 26.
R. O. Davies and G. O. Jones, Acta. Physica, 2 (1953) 370.
A. Q. Tool, J. Amer. Ceram. Soc., 29 (1946) 240.
W. Kauzmann, Chem. Rev., 48 (1948) 219.
C. T. Moynihan, A. J. Eastel and M. A. Debolt, J. Am. Ceram. Soc., 59 (1976) 12.
I. Prigogine and R. Defay, Thermodynamique Chimique, Vol. I-II, Desoer, Liège, Belgium 1950, p. 304.
P. Claudy, B. Bonnetot, G. Chahine and J. M. Létoffé, Thermochim. Acta, 38 (1980) 75.
E. L. Skau, J. Amer. Chem. Soc., 57 (1935) 243.
E. M. Barrall and R. D. Diller, Thermochim. Acta, 1 (1970) 509.
J. H. Flynn, Thermochim. Acta, 8 (1974) 69.
H. N. Ritland, J. Am. Ceram. Soc, 39 (1956) 403.
P. Claudy, J. C. Commerçon and J. M. Létoffé, Thermochim. Acta, 128 (1988) 251.
G. Tammann, Der Glaszustand, Section IV, 12, Leopold Voss, Leipzig 1933.
M. Hoare, Ann. New York Accademy Sci., 279 (1976) 187.
S. Jabrane, J. M. Létoffé, J. J. Counioux and P. Claudy, Thermochim. Acta, 290 (1996) 31.
P. Claudy, S. Jabrane and J. M. Létoffé, Thermochim. Acta, 293 (1996) 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Claudy, P., Siniti, M. & El Hajri, J. Thermodynamic Study of the Glass Relaxation Phenomena. DSC study of annealing of maltitol glass. Journal of Thermal Analysis and Calorimetry 68, 251–264 (2002). https://doi.org/10.1023/A:1014973719280
Issue Date:
DOI: https://doi.org/10.1023/A:1014973719280