Abstract
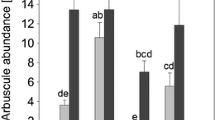
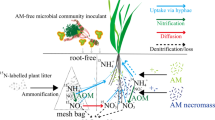
The effect of arbuscular mycorrhizae (AM) on soil microbial populations and on growth performance of the high salt marsh plant Spartina patens was investigated in a AM suppression study on field-collected soil cores with S. patens. The application of benomyl resulted in a significant reduction of AM colonization on roots of S. patens, but did not completely suppress AM. Non-treated cores had significantly greater colonization (26 ± 6%) than either benomyl- (12 ± 7%) or benomyl-phosphorus-treated (7 ± 3%) cores at a depth of 2.5 cm. Colonization differences between cores declined with depth (5.0 and 7.5 cm), however, so that at 7.5 cm there was no difference between treatments. This decline was attributed to a reduction in oxygen availability with depth as evidenced by decreasing redox potential. Basic environmental conditions generally resembled those found at the field site. There were no environmental differences between treatments at the depths examined. Cell numbers and specific biomass of DAPI-stained organisms as well as members of the Domain Bacteria were significantly higher when AM colonization was suppressed, while those of the Domains Eucarya and Archaea were not significantly influenced. The increase in both microbial and bacterial population size and biomass in the presence of lower levels of AM colonization is most likely due to increases in carbon exudation to soil and rhizosphere populations that accompany AM suppression. PCR-RFLP analysis of nifH amplicons in bulk soil and rhizosphere at varying depths through the soil cores showed differences in banding patterns between rhizosphere and soil material in the presence of AM. The lack of such strong differences in the benomyl-treated cores suggests that AM colonization more strongly affects the nitrogen-fixing population than do physicochemical conditions (e.g. redox potential) alone. Plant growth performance assessed by analyzing root and leaf biomass, as well as excitation transfer efficiency of open photosynthesis system II (PS II) reaction centers (Fv/Fm) was not significantly influenced by AM. Significant differences were found between treatments for C/N ratios and nitrogen content in leaf tissue, indicating that suppression of AM increased plant nitrogen acquisition.
Similar content being viewed by others
References
Adam P 1990 Salt marsh ecology. Cambridge University Press, Cambridge, UK.
Ames R N, Reid C P P, Porter L K and Cambardella C 1983 Hyphal uptake and transport of nitrogen from two N-labelled sources by Glomus mosseae, a vesicular-arbusuclar mycorrhizal fungus. New Phytol. 95, 381–396.
Armstrong Y 1978 Root aeration in the wetland condition. In Plant life in anaerobic environment. Eds. D E Hook and R M Crawford. pp 269–298. Ann Arbor Science Publ., Ann Arbor, MI, USA.
Bassam B J, Caetano-Anolles G and Gresshoff P M 1991 Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal. Biochem. 196, 80–83.
Bertness M D 1991 Zonation of Spartina patens and Spartina alterniflora in a New England salt marsh. Ecology 72, 138–148.
Bertness M D 1992 The ecology of a New England marsh. Amer. Sci. 80, 260–268.
Bertness M D and Callaway R 1994 Positive interactions in communities. Trends Ecol. Evol. 9, 191–193.
Callaway R M 1997 Positive interactions in plant communities and the individualistic-continuum concept. Oecologia, 143–149.
Chalmers A G 1979 The effects of fertilization on nitrogen distribution in a Spartina alterniflora salt marsh. Estuar. Coast. Shelf Sci. 36, 105–131.
Chatzinotas A, Sandaa R-A, Schönhuber W, Amann RI, Daae FL, Torsvik V, Zeyer J and Hahn D 1998 Analysis of broad-scale differences in microbial communities of two pristine forest soils. Syst. Appl. Microbiol. 21, 579–587.
Cooke J C, Butler R H and Madole G 1993 Some observations on the vertical distribution of vesicular arbuscular mycorrhizae in roots of salt marsh grasses growing in saturated soils. Mycologia 84, 547–550.
DeLaune R D, Feijtel T C and Patrick W H 1989 Nitrogen flows in a Louisiana gulf coast salt marsh: Spatial considerations. Biogeochemistry. 8, 25–37.
Edgcomb V P, McDonald J H, Devereux R and Smith D W 1999 Estimation of bacterial cell numbers in humic acid-rich salt marsh sediments with probes directed to 16S ribosomal DNA. Appl. Environ. Microbiol. 65, 1516–1523.
Fry J C 1990 Direct methods and biomass estimation. Meth. Microbiol. 22, 41–86.
Garbaye J 1991 Biological interactions in the mycorrhizosphere. Experientia 47, 370–375.
Gandy E L and Yoch D C 1988 Relationship between nitrogen-fixing sulfate reducers and fermenters in salt marsh sediments and roots of Spartina alterniflora. Appl. Environ. Microbiol. 54, 2031–2036.
Graham J H, and Eissenstat D M 1998 Field evidence for the carbon cost of citrus mycorrhizas. New Phytol. 140, 103–110.
Graham J H, Leonard R T and Menge J A 1981 Membrane mediated decrease in root exudation responsible for phosphorus inhibition of vesicular-arbuscular mycorrhizae formation. Plant Physiol. 68, 548–552.
Hahn D, Amann R I, Ludwig W, Akkermans A D L and Schleifer K-H 1992 Detection of microorganisms in soil after in situ hybridization with rRNA-targeted, fluorescently labelled oligonucleotides. J. Gen. Microbiol. 138, 879–887.
Hamel C, Nesser C, Barrantes-Cartin U, Furlan V and Smith D L 1991 Endomycorrhizal fungal species mediate 15N transfer from soybean to maize in non-fumigated soils. Plant Soil 138, 41–47.
Hanson R B 1977 Comparison of nitrogen fixation activity in tall and short Spartina alterniflora salt marsh soils. Appl. Environ. Microbiol. 33, 596–602.
Hart, M R and Brookes P C 1996 Soil microbial biomass and mineralization of soil organic matter after 19 years of cumulative field applications of pesticides. Soil Biol. Biochem. 28, 1641–1649.
Hayman D S 1986 Mycorrhizae of nitrogen-fixing legumes. MIRCEN J. 2, 121–145.
Hess A, Höhener P, Hunkeler D and Zeyer J 1996 Bioremediation of a diesel fuel-contaminated aquifer: Simulation studies in laboratory aquifer columns. J. Contam. Hydrol. 23, 329–345.
Hess A, Zarda B, Hahn D, Häner A, Stax D, Höhener P and Zeyer J 1997 In situ analysis of denitrifying toluene-and m-xylene-degrading bacteria in a diesel fuel-contaminated laboratory aquifer column. Appl. Environ. Microbiol. 63, 2136–2141.
Hetrick B A D, Harnett D C, Wilson G W T and Gibson D J 1994 Effects of mycorrhizae, phosphorus availability and plant density on yield relationships among competing tallgrass prairie grasses. Can. J. Bot. 72, 168–176.
Hoefnagels M H, Broome S W and Shafer S R 1993 Vesicular-arbuscular mycorrhizae in salt marshes in North Carolina. Estuaries 16, 851–858.
Hönerlage W, Hahn D and Zeyer J 1995 Detection of mRNA of nprM in Bacillus megaterium ATCC 14581 grown in soil by whole cell hybridization. Arch. Microbiol. 163, 235–241.
Jones H G 1992 Plants and microclimate. Cambridge University Press, Cambridge, UK.
Kahiluoto H, Ketoja E and Vestberg M 2000 Creation of a non-mycorrhizal control for a bioassay of AM effectiveness. 1. Comparison of methods. Mycorrhiza 9, 241–258.
Kahiluoto H and Vestberg M 2000 Creation of a non-mycorrhixal control for a bioassay of AM effectiveness. 2. Benomyl application and soil sampling time. Mycorrhiza 9, 259–270.
Kormanik P P and McGraw A-C 1982 Quantification of vesicular arbuscular mycorrhizae in plant roots. In Methods and Principals of Mycorrhizal Research. Ed. N C Schenk. pp 37–45. Amer. Phytopathol. Soc., St. Paul, USA.
Koroleff F 1983 Determination of ammonia. In Methods of Seawater Analysis. Eds. K Grasshoff, M Ehrhardt and K Kremling. pp 150–157. Verlag Chemie GmbH, Weinheim, Germany.
Larsen J, Thingstrup I, Jakobsen I and Rosendahl S 1996 Benomyl inhibits phosphorus transport not fungal alkaline phosphatase activity in Glomus-cucumber symbiosis. New Phytol. 132, 127–133.
Linderman R G 1988 Mycorrhizal interactions with the rhizosphere microflora: the mycorrhizosphere effect. Phytopath. 78, 366–371.
Moriarty D J W, Iverson R L and Pollard P C 1986 Exudation of organic carbon by the seagrass Halodule wrightii Aschers and its effect on bacterial growth in sediment. J. Exp. Mar. Biol. Ecol. 96, 115–126.
Olsson P A, Bååth E, Jakobsen I and Söderström B 1996 Soil bacteria respond to presence of roots but not to mycelium of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 28, 463–470.
Patriquin D G and Keddy C 1978 Nitrogenase activity (acetylene reduction) in a Nova Scotian salt marsh: Its association with angiosperms and the influence of some edaphic factors. Aquat. Bot. 4, 227–244.
Paul E A and Clark F E 1996 Soil Microbiology and Biochemistry. Academic Press, New York, USA.
Piceno Y M and Lovell C R 2000a Stability in natural bacterial communties: I. Nutrient addition effects on rhizosphere diazotroph assemblage composition. Microbiol. Ecol. 39, 32–40.
Piceno Y M and Lovell C R 2000b Stability in natural bacterial communties: II. Plant resource allocation effects on rhizosphere diazotroph assemblage composition. Microbiol. Ecol. 39, 41–48.
Piceno Y M, Noble P A and Lovell C R 1999 Spatial and temporal assessment of diazotroph assemblage composition in vegetated salt marsh sediments using denaturing gradient gel electrophoresis analysis. Microbiol. Ecol. 38, 157–167.
Rublee P A 1982. Bacteria and microbial distribution in estuarine sediments. In Estuarine Comparisons. Ed. V S Kennedy. pp 159–182. Academic Press, New York, USA.
Schmidt I K, Ruess L, Bååth E, Michelsen A, Ekelund F and Jonasson S 2000 Long-term manipulation of microbes and microfauna of two subarctic heaths by addition of fungicide, bactericide, carbon and fertilizer. Soil Biol. Biochem. 32, 707–720.
Schönholzer F, Hahn D and Zeyer J 1999 Origins and fate of fungi and bacteria in the gut of L. terrestris L. studied by image analysis. FEMS Microbiol. Ecol. 28, 235–248.
Schreiber U, Bilger W, Hormann H and Neubauer C 1998 Chlorophyll fluorescence as a diagnostic tool: Basics and some aspects of practical relevance. In Photosynthesis, A Comprehensive Treatise. Ed. A S Raghavendra. pp 320–336. Cambridge University Press, Cambridge, UK.
Smith G S 1988 The role of phosphorus nutrition in interactions of vesicular-arbuscular mycorrhizal fungi with soilborne nematodes and fungi. Phytopath. 78, 371–374.
Smith M D, Hartnett D C and Rice C W 2000 Effects of long-term fungicide applications on microbial properties in tallgrass prairie soil. Soil Biol. Biochem. 32, 935–946.
Smith S E and Read D J 1997 Mycorrhizal Symbiosis. Academic Press, San Diego, USA.
Sun Y-P, Unestam T, Lucas S D, Johanson K J, Kenne L and Finlay R 1999 Exudation-reabsorption in a mycorrhizal fungus, the dynamic interface for interaction with soil and soil microorganisms. Mycorrhiza 9, 137–144.
Valiela I and Teal J M 1974 Nutrient limitation of salt marsh vegetation. In Ecology of Halophytes. Eds. R J R Reimold and W H Queen. pp 547–563. Academic Press, New York, USA.
Valiela I and Teal J M 1979 The nitrogen budget of a salt marsh ecosystem. Nature 280, 652–656.
Van Duin W E, Rozema J and Ernst W H O 1989 Seasonal and spatial variation in the occurrence of vesicular-arbuscular mycorrhizae in salt marsh plants. Agricult. Ecosyst. Environ. 29, 107–110.
Welsh D T, Bourgues S, de Wit E and Herbert R A 1996 Seasonal variations in nitrogen-fixation (acetylene reduction) and sulfate-reduction rates in the rhizosphere of Zostera noltii: Nitrogen fixation by a sulfate-reducing bacteria. Mar. Biol. 125, 619–628.
White D S and Howes B L 1994 Translocation, remineralization, and turnover of nitrogen in the roots and rhizomes of Spartina alterniflora. Amer. J. Bot. 81, 1225–1234.
Whiting G J, Gandy E L and Yoch D C 1986 Tight coupling of root-associated nitrogen fixation and plant photosynthesis in the salt marsh grass Spartina alterniflora and carbon dioxide enhancement of nitrogenase activity. Appl. Environ. Microbiol. 52, 108–113.
Widmer F, Shaffer B T, Porteous L A and Seidler R J 1999. Analysis of nifH gene pool complexity in soil and Effects of an invasive reedgrass, Phragmites australis, on nitrogen cycling in brackish tidal marsh of New York and New Jersey. Ph.D. thesis, Rutgers University, New Brunswick, NJ, US. pp 170.
Wright D P, Scholes J D and Read D J 1998 Effects of VA mycorrhizal colonization on photosynthesis and biomass production of Trifolium repens L. Plant Cell Environ. 21, 209–216.
Zani S, Mellon M T, Collier J L and Zehr J P 2000 Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl. Environ. Microbiol. 66, 3119–3124.
Zarda B, Hahn D, Chatzinotas A, Schönhuber W, Neef A, Amann R I and Zeyer J 1997 Analysis of bacterial community structure in bulk soil by in situ hybridization. Arch.Microbiol. 168, 185–192.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burke, D.J., Hamerlynck, E.P. & Hahn, D. Effect of arbuscular mycorrhizae on soil microbial populations and associated plant performance of the salt marsh grass Spartina patens . Plant and Soil 239, 141–154 (2002). https://doi.org/10.1023/A:1014901518235
Issue Date:
DOI: https://doi.org/10.1023/A:1014901518235