Abstract
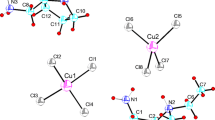
A new compound [Cu(C5H4NCOO)2] · 2H2O has been prepared. Its structure consists of a chain built from [Cu(C5H4NCOO)2] units, bridged through weak interactions between the copper(II) atoms and the O atoms of the carboxylate groups of a adjacent [Cu(C5H4NCOO)2] units. I.r. spectra, thermal analysis and elemental analysis have been recorded for the complex. Room temperature X-band e.s.r. spectra of powered samples and variable-temperature magnetic susceptibility studies indicate that the compound exhibits a weak ferromagnetic interaction through Cu—O—C—O—Cu pathways.
Similar content being viewed by others
References
R.B. King, Encyclopedia of Inorganic Chemistry, Wiley, New York, 1994.
M.M. Whittaker and J.W. Whittaker, Biophys. J., 64, 762 (1993).
J.P. Klinman, Chem. Rev., 96, 2541 (1996).
V. Mathrubootham, V. Rathinam, P. Mallayan, T. Balasubramanian, P. Prabharan and T.P. Muthiah, Inorg. Chem., 37, 6418 (1998).
H.R. Mahler and E.H. Cordes, Biological Chemistry, 2nd edit., Harper & Row, New York, 1971, p. 801.
A. Takenaka, H. Utsumi, T. Yamamoto, A. Furusaki and I. Nitta, Nippon Kagaku Zasshi., 91, 921 (1970).
N. Das and A.C. Dash, Indian J. Chem., 32A, 531 (1993).
O. Nobuo and K. Makiko, Acta Cryst., C54, 288 (1998).
P.R. Faure, H. Loiseleur and G. Thomas-David, Acta Cryst., B29, 1890 (1973).
G.M. Sheldrick, SHELXTL, Structure Determination Software Programs, Version 5.10. Bruker Analytical X-ray Systems Inc., Madison, Wisconsin, USA, 1997.
A.P. Ginsberg, Inorg. Chim. Acta Rev., 5, 45 (1971).
A. Earnshaw, Introduction to Magnetochemistry, Academic Press, London, 1968.
K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1970, p. 166.
K. Nakamoto, Infrared and Roman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1963, p. 233.
F.A. Cotton, G. Wilkinson, Advanced Inorganic Chemistry, 4th edit., Wiley, New York, p. 682.
M.A. Viswamitra, J. Chem. Phys., 37, 1408 (1962).
C. Friebel, G. Plesch, V. Kettmann, J. Krätsmar-Šmogrovic and O. Švajlenovc, Ionrg. Chim. Acta, 254, 273 (1997).
L.A. Kovbasyuk, I.O. Fritsky, V.N. Kokozay and T.S. Iskenderov, Polyhedron, 16, 1723 (1997).
F.S. Stephens, J. Chem. Soc., A, 2493 (1969).
E, Escrivá, J. Server-Carrio, L. Lezama, J.-V. Folgado, J.L. Pizarro, R. Baallesteros and B. Abarca, J. Chem. Soc., Dalton Trans., 2033 (1997) and refs. therein.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Luo, J.H., Hong, M.C., Shi, Q. et al. Synthesis, structure and magnetic properties of a quasi-two-dimensional compound [Cu(C5H4NCOO)2] · 2H2O. Transition Metal Chemistry 27, 311–315 (2002). https://doi.org/10.1023/A:1014876514184
Issue Date:
DOI: https://doi.org/10.1023/A:1014876514184