Abstract
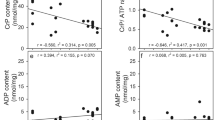
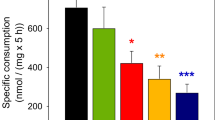
The metabolic effects of extracellular glutamine (2.5 mM) or high potassium (25 mM) on glucose metabolism were studied in cultured cerebellar astrocytes. High potassium caused an increased glycolytic flux and an increase in glutamine release. Exposure to glutamine increased glycolytic flux and alanine formation, indicating that glutamine uptake is an energy requiring process. The effects of glutamine and high potassium on glycolytic flux were additive. Formation of metabolites from [1-13C]glucose and [2-13C]acetate confirmed the effects of glutamine and high potassium on glycolytic metabolism. In the presence of extracellular glutamine, analysis of the 13C labeling patterns of citrate and glutamine indicated a decrease in the cycling ratio and/or pyruvate carboxylation and glutamine synthesis from [1-13C]glucose did occur, but was decreased. Exposure to high potassium led to extracellular accumulation of acetate, presumably through non-enzymatic decarboxylation of pyruvate.
Similar content being viewed by others
REFERENCES
Sokoloff, L. 1977. Relation between physiological function and energy metabolism in the central nervous system. J. Neurochem. 29:13–26.
Tsacopoulos, M. and Magistretti, P. J. 1996. Metabolic coupling between glia and neurons. J. Neurosci. 16:877–885.
Peng, L., Zhang, X., and Hertz, L. 1994. High extracellular potassium concentrations stimulate oxidative metabolism in a glutamatergic neuronal culture and glycolysis in cultured astrocytes but have no stimulatory effect in a GABAergic neuronal culture. Brain Res. 663:168–172.
Pellerin, L. and Magistretti, P. J. 1994. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 91:10625–10629.
Qu, H., Eloqayli, H., Unsgåard, G., and Sonnewald, U. 2001. Glutamate Decreases Pyruvate Carboxylase Activity and Spares Glucose as Energy Substrate in Cultured Cerebellar Astrocytes. J. Neurochem. Res. In press.
Van den Berg, C. J. and Garfinkel, D. 1971. A simulation study of brain compartments. Metabolism of glutamate and related substances in mouse brain. Biochem. J. 123:211–218.
Waagepetersen, H. S., Bakken, I. J., Larsson, O. M., Sonnewald, U., and Schousboe, A. 1998. Comparison of lactate and glucose metabolism in cultured neocortical neurons and astrocytes using 13C-NMR spectroscopy. Dev. Neurosci. 20:310–320.
Benjamin, A. M. and Quastel, J. H. 1975. Metabolism of amino acids and ammonia in rat brain cortex slices in vitro: a possible role of ammonia in brain function. J. Neurochem. 25:197–206.
Bradford, H. F., Ward, H. K., and Thomas, A. J. 1978. Glutamine-a major substrate for nerve endings. J. Neurochem. 30:1453–1459.
Hassel, B., Sonnewald, U., and Fonnum, F. 1995. Glial-neuronal interactions as studied by cerebral metabolism of [2–23C]acetate and [1–13C]glucose: an ex vivo 13C NMR spectroscopic study. J. Neurochem. 64:2773–2782.
Yudkoff, M., Nissim, I., and Pleasure, D. 1988. Astrocyte metabolism of [15N]glutamine: implications for the glutamineglutamate cycle. J. Neurochem. 51:843–850.
Hassel, B., Sonnewald, U., Unsgard, G., and Fonnum, F. 1994. NMR spectroscopy of cultured astrocytes: effects of glutamine and the gliotoxin fluorocitrate. J. Neurochem. 62:2187–2194.
Sonnewald, U., Westergaard, N., Jones, P., Taylor, A., Bachelard, H. S., and Schousboe, A. 1996. Metabolism of [U-13C5] glutamine in cultured astrocytes studied by NMR spectroscopy: first evidence of astrocytic pyruvaterecycling. J. Neurochem. 67:2566–2572.
Broer, A., Brookes, N., Ganapathy, V., Dimmer, K. S., Wagner, C. A., Lang, F., and Broer, S. 1999. The astroglial ASCT2 amino acid transporter as a mediator of glutamine efflux. J. Neurochem. 73:2184–2194.
Hertz, L., Juurlink, B. H. J., Hertz, E., Fosmark, H., and Schousboe, A. 1989. Preparation of primary cultures of mouse (rat): Astrocytes. In: A. Shahar, J. De Vellis, A. Vernadakis, and B. Haber (Eds.) A Dissection and Tissue Culture Manual for the Nervous System, Alan R Liss, New York, pp. 105–108.
Hassel, B., Bachelard, H., Jones, P., Fonnum, F., and Sonnewald, U. 1997. Trafficking of amino acids between neurons and glia in vivo. Effects of inhibition of glial metabolism by fluoroacetate. J. Cereb. Blood Flow Metab. 17:1230–1238.
Chaudhry, F. A., Reimer, R. J., Krizaj, D., Barber, D., Storm-Mathisen, J., Copenhagen, D. R., and Edwards, R. H. 1999. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell 99:769–780.
Fei, Y. J., Sugawara, M., Nakanishi, T., Huang, W., Wang, H., Prasad, P. D., Leibach, F. H., and Ganapathy, V. 2000. Primary structure, genomic organization, and functional and electrogenic characteristics of human system N 1, a Na+-and H+-coupled glutamine transporter. J. Biol. Chem. 275:23707–23717.
Nakanishi, T., Sugawara, M., Huang, W., Martindale, R. G., Leibach, F. H., Ganapathy, M. E., Prasad, P. D., and Ganapathy, V. 2001. Structure, function, and tissue expression pattern of human SN2, a subtype of the amino acid transport system N. Biochem. Biophys. Res. Commun. 281:1343–1348.
Laake, J. H., Takumi, Y., Eidet, J., Torgner, I. A., Roberg, B., Kvamme, E., and Ottersen, O. P. 1999. Postembedding immunogold labelling reveals subcellular localization and pathway-specific enrichment of phosphate activated glutaminase in rat cerebellum. Neuroscience. 88:1137–1151.
O'Donnell-Tormey, J., Nathan, C. F., Lanks, K., DeBoer, C. J., and de la Harpe, J. 1987 Secretion of pyruvate. An antioxidant defense of mammalian cells. J. Exp. Med. 165:500–514.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hassel, B., Sonnewald, U. Effects of Potassium and Glutamine on Metabolism of Glucose in Astrocytes. Neurochem Res 27, 167–171 (2002). https://doi.org/10.1023/A:1014827327690
Issue Date:
DOI: https://doi.org/10.1023/A:1014827327690