Abstract
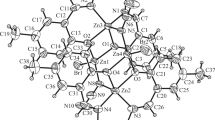
Copper(II) complexes of general formula, Cu(NNS)X 2 · nH2O (NNS = the 2-formylpyridine Schiff base of N-methyl-S-methyldithiocarbazate; X = Cl−, Br−, I−, NCS−; n = 0, 2) have been synthesized and characterized by elemental analysis and by magnetic and spectroscopic techniques. Based on magnetic and spectroscopic data, a monomeric five-coordinate square-pyramidal structure is assigned to these complexes. The crystal and molecular structure of [Cu(NNS)I2] has been determined by X-ray diffraction. The complex has a monomeric square-pyramidal structure with the ligand coordinated to the copper(II) ion via the pyridine nitrogen atom, the azomethine nitrogen atom and the thione sulfur atom. The fourth and fifth coordination sites are occupied by the iodide ligands. Antimicrobial tests indicate that Schiff base is inactive against the bacteria, Bacillus subtilis (mutant defective DNA repair), Pseudomonas aeruginosa, methicillin resistant Staphylococcus aureus and Bacillus subtilis (wild type) and weakly active against the fungi, Candida albicans, Candida lypolytica, Saccharomyces cereviseae and Aspergillus ochraceous but its copper(II) complexes, Cu(NNS)X 2 are strongly active against these organisms. A cytotoxicity study of the compounds against leukemic and cervical cancer cells showed that the Schiff base is inactive, but the complexes, [Cu(NNS)I2] and [Cu(NNS)(NCS)2] · 2H2O exhibit significant activity against cervical cancer cells with CD50 values of 4.8 and 4.2 μg, respectively.
Similar content being viewed by others
References
M. Akbar Ali, R.J. Butcher and J.C. Bryan, Inorg. Chim. Acta, 287, 8 (1999).
M. Akbar Ali, M. Nazimuddin, R. Saha, R.J. Butcher and J.C. Bryan, Polyhedron, 17, 3955 (1998).
M. Akbar Ali, S.M.M.H. Majumder, R.J. Butcher, J.P. Jasinski and J.M. Jasinski, Polyhedron, 16, 2749 (1997).
M.E. Hossain, M.N. Alam, M. Akbar Ali, M. Nazimuddin, F.E. Smith and R.C. Hynes, Polyhedron, 15, 973 (1996).
M.E. Hossain, M.N. Alam, J. Begum, M. Akbar Ali, M. Nazimuddin, F.E. Smith and R.C. Hynes, Inorg. Chim. Acta, 249, 207 (1996).
M. Akbar Ali, T.S. Guan, P. Bhattacharjee, R.J. Butcher, J.P. Jasinski and Yu Li, Transition Met. Chem., 21, 351 (1996).
M. Akbar Ali, K.R. Fernando, D. Palit and M. Nazimuddin, Transition Met. Chem., 20, 19 (1995).
M. Rahman and M. Akbar Ali, Transition Met. Chem., 19, 237 (1994).
M. Rahman and M. Akbar Ali, Transition Met. Chem., 18, 396 (1993).
M.E. Hossain, M.N. Alam, J. Begum, M. Akbar Ali and M. Nazimuddin, Transition Met. Chem., 18, 497 (1993).
M. Akbar Ali and S.E. Livingstone, Coord. Chem. Rev., 13, 101 (1974) and refs. cited therein.
A. Abu-Raqabah, G. Davies, M.A. El-Sayed, A. El-Toukly, S.N. Shaikh and J. Zubeita, Inorg. Chim. Acta, 193, 43 (1992).
K.D. Onan, G. Davies, M.A. El-Sayed, and A. El-Toukly, Inorg. Chim. Acta, 119, 121 (1986).
M. Das and S.E. Livingstone, Br. J. Cancer, 37, 101 (1978).
M. Akbar Ali, G. Hossain, S.M.M.H. Majumder and M. Nazimuddin, Polyhedron, 6, 1653 (1987).
M. Akbar Ali, S.E. Livingstone and D.J. Philips, Inorg. Chim. Acta, 6, 11 (1972).
M.T.H. Tarafder, M.A. Ali, D.J. Wee, K. Azahari, S. Silong and K.A. Crouse, Transition Met. Chem., 25, 456 (2000).
G.M. Sheldrick, SHELXTL, Crystallographic Systems (Siemens Analytical Instruments Division), Madison, Wisconsin, USA, (1986).
D.T. Cromer and J.T. Weber, Table 2.2, International Tables for X-ray Crystallography, Vol. IV, The Kynoch Press, Birmingham, England, 1974.
D.T. Cromer, Table 2.3.1, International Tables for X-ray Crystallography, Vol. IV, The Kynoch Press, Birmingham, England, 1974.
G.M. Sheldrick, SHELTXL 96, Acta Crystallogr. A 46, 467 (1990).
R.A. Bailey, S.L. Kojak, T.W. Michelson and M.N. Mills, Coord. Chem. Rev., 6, 1407 (1971).
J. Garcia-Tojal, J.G. Jaca, R. Cortes, T. Rojo, M.K. Urtiaga and M.T. Arriortua, Inorg. Chim. Acta, 249, 25 (1996).
A.G. Bingham, H. Bogge, A. Muller, E.W. Ainscough and A.M. Brodie, J. Chem. Soc., Dalton Trans., 493 (1987); E.W. Ainscough, A.M. Brodie and N.G. Larson, Inorg. Chim. Acta, 60, 25 (1982); E.W. Ainscough, A.M. Brodie and N.G. Larsen, J. Chem. Soc., Dalton Trans., 815 (1982).
U. Sakaguchi and A.W. Addisons, J. Chem. Soc., Dalton Trans., 600 (1979).
P. Comba, N.F. Curtis, G.A. Lawrence, A.M. Sargeson, B.U. Skelton and A.H. White, Inorg. Chem., 25, 4240 (1986); K. Miyoshi, H. Tanaka, E. Kimura, S. Tsuboyama, S. Murata, D.H. Shimizu and K. Ishizu, Inorg. Chim. Acta, 78, 23 (1983); B.J. Hathway, Coord. Chem. Rev., 41, 423 (1982).
M.J. Bew, B.J. Hatta and R.J. Fereday, J. Chem. Soc., Dalton Trans., 29 (1972).
M.R. Taylor, J.P., Glusker, E.J. Gabe and J.A. Minkin, Bioinorg. Chem., 3, 189 (1974).
A.C. Villa, A.G. Manfredotti and G. Guastini, Cryst. Struct. Commun., 1, 125 (1972).
C. O'sullivan, G. Murphy, B. Murphy and B.J. Hathaway, J. Chem. Soc., Dalton Trans., 1835 (1999).
A.W. Addison, T.N. Rao, J. Reedjik, J. Van Rijn and G.C. Verschoor, J. Chem. Soc., Dalton Trans., 1345 (1984).
J.S. Casas, M.V. Castano, Garcia-Tasende, I. Martinez-Santamarta, J. Sordo, E.E. Castellano and J. Zukerman-Schpector, J. Chem. Res. (S), 324 (1992).
A.M. Ali, M.M. Mackeen, I.I. Safinar, M. Hamid, N.H. Lajis, S.H. El-sharkawy and M. Murakoshi, J. Ethnopharmacology, 53, 165 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akbar Ali, M., Huq Mirza, A., Haji Iran, M.S. et al. Magnetic, spectroscopic and biological properties of copper(II) complexes of the tridentate ligand, α-N-methyl-S-methyl-β-N-(2-pyridyl)methylenedithiocarbazate(NNS) and the X-ray crystal structure of the [Cu(NNS)I2] complex. Transition Metal Chemistry 27, 262–267 (2002). https://doi.org/10.1023/A:1014813315980
Issue Date:
DOI: https://doi.org/10.1023/A:1014813315980