Abstract
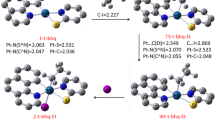
The mechanism of nucleophilic addition of oximes to organic nitriles coordinated to platinum was studied by ab initio methods of quantum chemistry using trans-[PtCl4(NCCH3)2] as an example. It was shown that in the absence of acidic or basic catalysis, the reaction proceeds through the formation of an orientation complex and a 4-membered cyclic transition state, whose decomposition yields the product of oxime addition to the C≡N bond. To compare and elucidate the reasons for nitrile activation in these complexes, the mechanism of hypothetical addition of formaldoxime to noncoordinated acetonitrile was studied. Calculated values of activation energy and energy effects of the reactions allow one to interpret the activation of nitriles during complexation in terms of the activated-complex model.
Similar content being viewed by others
REFERENCES
Kukushkin, Yu.N., Reaktsionnaya sposobnost' koordinatsionnykh soedinenii (Reaction Capacity of Coordination Compounds), Leningrad: Khimiya, 1987.
Kukushkin, Yu.N., Khimiya koordinatsionnykh soedinenii (Chemistry of Coordination Compounds), Moscow: Vysshaya Shkola, 1985.
Kukushkin, V.Yu., Zenkevich, I.G., Belsky, V.K., et al., Inorg. Chim. Acta, 1989, vol. 166, p. 79.
Michelin, R.A., Mozzon, M., and Bertani, R., Coord. Chem. Rev., 1996, vol. 147, p. 299.
Cini, R., Fanizzi, F.P., Intini, F.P., et al., Inorg. Chim. Acta, 1996, vol. 251, nos. 1-2; p. 111.
Cini, R., Fanizzi, F.P., Intini, F.P., et al., Inorg. Chim. Acta, 1997, vol. 264, nos. 1-2, p. 279.
Cini, R., Caputo, P.A., Intini, F.P., and Natile, G., Inorg. Chem., 1995, vol. 34, no. 5, p. 1130.
Ros, R., Renaud, J., and Roulet, R., J. Organomet. Chem., 1976, vol. 104, p. 271.
Kukushkin, Yu.N. and Larionova, Yu.E., Zh. Obshch. Khim., 1994, vol. 64, no. 9, p. 1409.
Ang, H.-G., Koh, C.-H., Koh, L.-L., et al., J. Chem. Soc., Dalton Trans., 1993, no. 6, p. 847.
Cotton, F.A., Daniels, L.M., Murillo, C.A., and Wang, X., Polyhedron, 1998, vol. 17, no. 17, p. 2781.
Cotton, F.A. and Kuhn, F.E., J. Am. Chem. Soc., 1996, vol. 118, no. 24, p. 5826.
Albertin, A., Antoniutti, S., Bacchi, A., et al., Inorg. Chem., 1998, vol. 37, no. 3, p. 479.
Wagner, G., Pombeiro, A.J.L., Bokach, N.A., and Kukushkin, V.Yu., J. Chem. Soc., Dalton Trans., 1999, no. 22, p. 4083.
Kukushkin, V.Yu., Ilichev, I.V., Wagner, G., et al., J. Chem. Soc., Dalton Trans., 1999, no. 17, p. 3047.
Kukushkin, V.Yu., Ilichev, I.V., Zhdanova, M.A., et al., J. Chem. Soc., Dalton Trans., 2000, no. 10, p. 1567.
Fereira, C.M.P., Guedes da Silva, M.F.C., Frausto da Silva, J.J.R., et al., Inorg. Chem., 2001, vol. 40, no. 6, p. 1134.
Panina, N.S. and Kukushkin, Yu.N., Zh. Neorg. Khim., 1998, vol. 43, no. 3, p. 469.
Kuznetsov, M.L., Dement'ev, A.I., Shestakova, O.S., and Kukushkin, V.Yu., Zh. Neorg. Khim., 2001, vol. 46, no. 10, p. 1683.
Kuznetsov, M.L., Bokach, N.A., Kukushkin, V.Yu., et al., J. Chem. Soc., Dalton Trans., 2000, no. 24, p. 4683.
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., et al., J. Comput. Chem., 1993, vol. 14, p. 1347.
Andrae, D., Haeussermann, U., Dolg, M., et al., Theor. Chim. Acta, 1990, vol. 77, p. 123.
Ditchfield, R., Hehre, W.J., and Pople, J.A., J. Chem. Phys., 1971, vol. 54, no. 2, p. 724.
Hehre, W.J., Ditchfield, R., and Pople, J.A., J. Chem. Phys., 1972, vol. 56, no. 5, p. 2257.
Francl, M.M., Pietro, W.J., Hehre, W.J., et al., J. Chem. Phys., 1982, vol. 77, no. 7, p. 3654.
Fukui, K., Acc. Chem. Res., 1981, vol. 14, no. 12, p. 363.
Gonzalez, C. and Schlegel, H.B., J. Chem. Phys., 1991, vol. 95, no. 8, p. 5853.
Kukushkin, V.Yu., Pakhomova, T.B., Kukushkin Yu., N., et al., Inorg. Chem., 1998, vol. 37, no. 25, p. 6511.
Kukushkin, V.Yu., Pakhomova, T.B., Bokach, N.A., et al., Inorg. Chem., 2000, vol. 39, no. 2, p. 216.
Garnovskii, D.A., Guedes da Silva, M.F.C., Pakhomova, T.B., et al., Inorg. Chim. Acta, 2000, vols. 300-302, p. 499.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kuznetsov, M.L., Dement'ev, A.I. Theoretical Study of the Mechanism of Nucleophilic Addition of Oximes to trans-[PtCl4(NCCH3)2]. Russian Journal of Coordination Chemistry 28, 191–200 (2002). https://doi.org/10.1023/A:1014776019049
Issue Date:
DOI: https://doi.org/10.1023/A:1014776019049