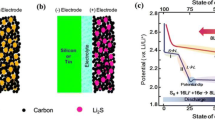
Abstract
Cycling batteries with cathodes based on elementary sulfur in 0.1 M LiClO4 solution in sulfolane leads to a decrease in the depth of cathodic reduction of sulfur and the anodic oxidation of the reduction products. Increasing the polarization current density diminishes the cycling depth and efficiency.
Similar content being viewed by others
REFERENCES
Marmorstein, D., Yu, T.H., Striebel, K.A., et al., J. Power Sources, 2000, vol. 89, p. 219.
Tobishima, S.-I., Yamamoto, H., and Matsuda, M., Electrochim. Acta, 1997, vol. 42, p. 1019.
Bikbaeva, G.G., Gavrilova, A.A., and Kolosnitsyn, V.S., Elektrokhimiya, 1993, vol. 29, p. 716.
Bikbaeva, G.G., Gavrilova, A.A., and Kolosnitsyn, V.S., Elektrokhimiya, 1994, vol. 30, p. 760.
Badoz-Lambling, J., Bonnaterret, R., Cauquis, G., et al., Electrochim. Acta, 1976, vol. 21, p. 119.
Rauh, R.D., Shuker, F.S., Marston, J.M., and Brummer, S.B., J. Inorg. Nucl. Chem., 1977, vol. 39, p. 1761.
Paris, J., Electrochim. Acta, 1981, vol. 26, p. 1823.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kolosnitsyn, V.S., Karaseva, E.V., Amineva, N.A. et al. Cycling Lithium–Sulfur Batteries. Russian Journal of Electrochemistry 38, 329–331 (2002). https://doi.org/10.1023/A:1014755428523
Issue Date:
DOI: https://doi.org/10.1023/A:1014755428523