Abstract
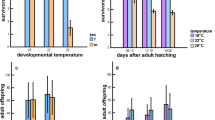
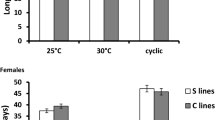
The genetic response of body size to temperature in the laboratory provides an interesting example of phenotypic plasticity. We found that females of Drosophila ananassae reared to adulthood at 18°C showed significant increase in body weight as compared to females reared at 25°C. At a given temperature, early productivity and lifetime productivity were the highest when the rearing and test temperature were the same. The effect of test temperature was highly significant for total productivity and early productivity. The interaction between test temperature and development temperature was also highly significant. Effect of development temperature was not significant. The females reared at 18°C showed greater body weight but their productivity was not significantly higher than smaller females reared at 25°C. Thus, the usually close relationship between size and fecundity is lost when the size change is due to rearing temperature. These findings provide evidence for adaptive plasticity in D. ananassae. We also found a negative correlation (trade-off) between longevity and productivity, the first report of such a trade-off between longevity and productivity in D. ananassae.
Similar content being viewed by others
References
Betran, E., M. Santos & A. Ruiz, 1998. Antagonistic pleiotropic effect of second-chromosome inversions on body size and early life-history traits in Drosophila buzzatii. Evolution 52: 144–154.
Callahan, S., M. Pigliucci & D. Schlichting, 1997. Developmental phenotypic plasticity: where ecology and evolution meet molecular biology. Bioessay 19: 519–525.
Capy, P., E. Pla & J.R. David, 1993. Phenotypic and genetic variability of morphometrical traits in natural populations of Drosophila melanogaster and D. simulans. I. Geographic variations. Genet. Sel. Evol. 25: 517–536.
Capy, P., E. Pla & J.R. David, 1994. Phenotypic and genetic variability of morphometrical traits in natural populations of Drosophila melanogaster and D. simulans. II. Within population variability. Genet. Sel. Evol. 26: 15–18.
Charlesworth, B., 1980. Evolution in Age-structured Populations. Cambridge University Press, Cambridge.
Chippendale, A.K., A.M. Leroi, S.B. Kim & M.R. Rose, 1993. Phenotypic plasticity and selection in Drosophila life history evolution. I. Nutrition and the cost of reproduction. J. Evol. Biol. 6: 171–193.
Cohet, Y., J. Vouidibio & J.R. David, 1980. Thermal tolerance and geographic distribution: a comparison of cosmopolitan and tropical endemic Drosophila species. J. Therm. Biol. 5: 69–74.
David, J.R. & C. Bocquet, 1975. Similarities and differences in latitudinal adaptation of two Drosophila sibling species. Nature 257: 588–590.
David, J.R. & L. Tsacas, 1981. Cosmopolitan, subcosmopolitan and widespread species: different strategies within the drosophilid family. Comptes Rendus Soc. Biogeogr. 57: 11–26.
David, J.R., R. Allemand, J. van Herrewege & J. Cohet, 1983. Ecophysiology: abiotic factors pp.106–154 in The Genetics and Biology of Drosophila, Vol. 3d, edited by M. Ashburner, H.L. Carson & J.N. Thompson Jr. Academic Press, New York.
Gotthard, K. & S. Nylin, 1985. Adaptive plasticity and plasticity as an adaptation: a selective review of plasticity in animal morphology and life history. Oikos 74: 3–17.
Huey, R.B., T. Wakefield, W.D. Crill & G.W. Gilchrist, 1995. Within-and between-generation effect of temperature on early fecundity of Drosophila melanogaster. Heredity 74: 216–223.
Jones, J.S., A. Coyne & L. Partridge, 1987. Estimation of the thermal niche of Drosophila melanogaster using a temperature sensitive mutation. Am. Nat. 130: 83–90.
Leroi, A.M., A.K. Chippendale & M.R. Rose, 1994. Long term laboratory evolution of a genetic life history trade-off in Drosophila melanogaster. 1. The role of genotype-by-environment interaction. Evolution 48: 1244–1257.
Mayr, E., 1966. Animal Species and Evolution. Belknap Press, Harvard.
McCabe, J. & L. Partridge, 1997. An interaction between environmental temperature and genetic variation for body size for the fitness of adult female Drosophila melanogaster. Evolution 51: 1164–1174.
Morin, J.P., B. Moreteau, G. Petavy, R. Parkash & J.R. David, 1997. Reaction norms of morphological traits in Drosophila: adaptive shape changes in a stenotherm circumtropical species? Evolution 51: 1140–1148.
Nunney, L., 1996. The response to selection for fast larval development in Drosophila melanogaster and its effect on adult weight: an example of a fitness trade-off. Evolution 50: 1193–1204.
Nunney, L. & W. Cheung, 1997. The effect of temperature on body size and fecundity in female Drosophila melanogaster: evidence for adaptive plasticity. Evolution 51: 1529–1535.
Parker, G.A. & J.M. Smith, 1990. Optimality theory in evolutionary biology. Nature 348: 27–33.
Partridge, L., K. Fowler, S. Trevitt & W. Sharp, 1986. An examination of the effect of males on the survival and egg production rates of female Drosophila melanogaster. J. Insect Phys. 32: 925–929.
Partridge, L., B. Barrie., N.H. Barton, K. Fowler & V. French, 1995. Rapid laboratory evolution of adult life history traits in Drosophila melanogaster in response to temperature. Evolution 49: 538–544.
Robertson, F.W., 1957. Studies in quantitative inheritance. XI. Genetic and environmental correlation between body size and egg production in Drosophila melanogaster. J. Genet. 55: 428–443.
Rose, M.L., 1991. Evolutionary Biology of aging. Oxford University Press. Oxford.
Singh, B.N., 1985. Drosophila ananassae — a genetically unique species. Nucleus 28: 169–176.
Singh, B.N., 1996. Population and behaviour genetics of Drosophila ananassae. Genetica 97: 321–332.
Singh, B.N., 2000. Drosophila ananassae — a species characterised by several unusual genetic features. Curr. Sci. 78: 391–398.
Singh, B.N. & S. Mathew, 1996. Greater mating success of Drosophila ananassae flies possessing high number of sternopleural bristles. Curr. Sci. 70: 1088–1089.
Singh, B.N. & S. Mathew, 1997. Greater fertility of Drosophila ananassa flies posessing high number of sternopleural bristles. Curr. Sci. 72: 112–114.
Singh, B.N. & S.R. Singh, 2001. Female remating in Drosophila ananassae: evidence for sperm displacement and greater productivity after remating. Zool. Sci. 18: 181–185.
Stearns, S.C., 1989. Trade-offs in life history evolution. Func. Ecol. 3: 259–268.
Stearns, S.C., 1992. The Evolution of Life Histories. Oxford University Press, Oxford.
Stearns, S.C., M. Ackermann, M. Doebeli & M. Kaiser, 2000. Experimental evolution of aging, growth, and reproduction in fruitflies. Proc. Natl. Acad. Sci. USA 97: 3309–3313.
Tantawy, A.O. & F.A. Rakha, 1964. Studies on natural populations of Drosophila. IV. Genetic variances of and correlations between four characters in Drosophila melanogaster and Drosophila simulans. Genetics 50: 1349–1355.
Williams, G.C., 1957. Pleiotropy, natural selection and the evolution of senescence. Evolution 11: 398–411.
Zamudio, K.R., R.B. Huey & W.D. Crill, 1995. Bigger isn't always better: body size developmental and parental temperature and male territorial success in Drosophila melanogaster. Anim. Behav. 49: 671–677.
Zwaan, B.J., R. Bijlsma & R.F. Hoekstra, 1995a. Artificial selection for development time in Drosophila melanogaster in relation to aging: direct and correlated responses. Evolution 49: 635–648.
Zwaan, B.J., R. Bijlsma & R.F. Hoekstra, 1995b. Direct selection on lifespan in Drosophila melanogaster. Evolution 49: 649–659.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sisodia, S., Singh, B.N. Effect of Temperature on Longevity and Productivity in Drosophila Ananassae: Evidence for Adaptive Plasticity and Trade-Off Between Longevity and Productivity. Genetica 114, 95–102 (2002). https://doi.org/10.1023/A:1014640604740
Issue Date:
DOI: https://doi.org/10.1023/A:1014640604740