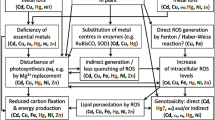
Abstract
Glutathione pool and redox status, as well as chlorophyll fluorescencewere measured in Tortula ruralis which was treated withheavy metals and exposed to different desiccation treatments. Two hours afterre-wetting, the ratio of oxidised glutathione to total glutathione poolreturnedto the steady state level (14%) in slowly dried unpolluted plants. Cdtreatment doubled this ratio, as did rapid drying without the heavy metaltreatment. When Cd and rapid drying were applied together, the ratio of GSSGreached 45% indicating a clear additive effect of these two stressfactors. RFd, a chlorophyll fluorescence parameter followed a similar pattern.Lead did not cause the depletion of the glutathione pool but increased theratioof GSSG. It is suggested that Cd and rapid desiccation exert their damageadditively. This might also entail a lowered degree of desiccation tolerance inareas polluted with metals and therefore a retreat of the mosses to mesicmicrohabitats.
Similar content being viewed by others
References
Anderson A. 1985. Determination of glutathione and glutathion disulfide in biological samples. Method Enzimol. 113: 548–555.
Brennan R.J. and Schiestl R.H. 1996. Cadmium is an inducer of oxidative stress in yeast. Mutation Research-Fundam. Mol. Mechanism Mutagenesis. 356: 171–178.
Brown D.H. and Beckett R.P. 1985. The role of the cell wall in the intracellular uptake of cations by lichens. In: Brown D.H. (ed.), Lichen physiology and cell biology. Plenum Press, New York, pp. 247–258.
Bruns I., Friese K., Markert B. and Krauss G.J. 1997. The use of Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals. 2. Heavy metal accumulation and physiological reaction of Fontinalis antipyretica L. ex Hedw. in active biomonitoring in the River Elbe. Sci. Tot. Enviro. 204: 161–176.
Dhindsa R.S. 1987. Glutathione status and protein synthesis during drought and subsequent rehydration in Tortula ruralis. Plant Physiol. 83: 816–819.
Dhindsa R.S. 1991. Drought stress, enzymes of glutathione metabolism, oxidation injury, and protein synthesis in Tortula ruralis. Plant Physiol. 95: 648–651.
Gallego S.M., Benavides M.P. and Tomaro M.L. 1996. Effect of heavy metal ion excess on sunflower leaves: Evidence for involvement of oxidative stress. Plant Sci. 121: 151–159.
Horváth G., Droppa M., Horváth L.I. and Szalontai B. 1995. Effects of heavy metal induced stress on the photosynthetic membrane characteristics. Acta Phytopat. Entom. Hung. 30: 127–129.
Jackson P.P., Robinson J. and Whitton B.A. 1991. Low molecular weight metal complexes in the freshwater moss Rhynchostegium riparioides exposed to elevated concentrations of Zn, Cu, Cd and Pb in the laboratory and field. Environ. Exp. Bot. 31: 359–366.
Koeppe D.E. 1981. Lead: Understanding the minimal toxicity of lead in plants. In: Lepp N.W. (ed.), Effect of heavy metal pollution on plants. Vol. 1. Applied Science Publishers, London and New Jersey, pp. 77–110.
Kranner I. and Grill D. 1997. Desiccation and the subsequent recovery of cryptogamics that are resistant to drought. Phyton-Annales Rei Botanicae. 37: 139–150.
Kranner I. and Lutzoni F. 1999. Evolutionary Consequences of transition to a lichen symbiotic state and physiological adaptation to oxidative damage associated with poikiloydry. In: Lerner H.R. (ed.), Plant responses to environmental stresses-from phytohormones to genome reorganisation. Marcel Dekker, Inc, New York, Basel, pp. 591–628.
Krupa Z. and Baszynszki T. 1995. Some aspects of heavy metal toxicity towards photosynthetic apparatus-direct and indirect effects on light and dark reactions. Acta Physiol. Plant. 17: 177–190.
Lichtenthaler H.K. 1996. Vegetation stress: an introduction to the stress concept in plants. J. Plant Physiol. 148: 4–14.
Oliver M.J. 1996. Desiccation tolerance in vegetative plant-cells. Physiol Plant. 97: 779–787.
Park K.S., Kwon J. and Choi S.Y. 1998. Cloning, characterization, and expression of the CIP2 gene induced under cadmium stress in Candida sp. Fems Microbiol. Lett. 162: 325–330.
Seel W.E., Hendry G.A.F. and Lee J.A. 1992. Effects of desiccation on some activated oxygen processing enzymes and antioxidants in mosses. J. Exp. Bot. 43: 1031–1037.
Senaratna T., McKersie B.D. and Borochov A. 1987. Desiccation and free radical mediated changes in plant membranes. J. Exp. Bot. 38: 2005–2014.
Smirnoff N. 1993. The role of active oxigen in the response of plants to water deficit and desiccation. Tansley Review No. 52, New Phytol. 125: 27–58.
Stephen D.W.S. and Jamieson D.J. 1997. Amino acid-dependent regulation of the Saccharomyces cerevisiae GSH1 gene by hydrogene peroxide. Mol. Microbiol. 23: 203–210.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takács, Z., Tuba, Z. & Smirnoff, N. Exaggeration of desiccation stress by heavy metal pollution in Tortula ruralis: a pilot study. Plant Growth Regulation 35, 157–160 (2001). https://doi.org/10.1023/A:1014481704892
Issue Date:
DOI: https://doi.org/10.1023/A:1014481704892