Abstract
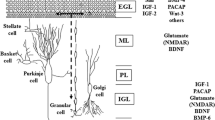
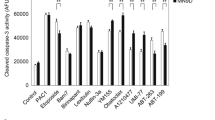
To provide an explanation for earlier paradoxical findings of lithium on survival of mature and immature neurons, this study monitors changes in cytosolic caspases in rat cerebellar granule cells (CGC) grown 2–7 days in vitro (DIV), or in murine E-17 cortical neurons. Data show Li+ protects mature 7-DIV CGC parallel to a decrease in proximal and distal caspases but increases levels for immature 2-DIV-CGC or E-17 cortical neurons. Caspases mirror viability based on morphological analyses (dye uptake, phase-contrast, DNA fragmentation), and suggest protection occurs by suppressing activation of a cascade resulting in distal effectors that destroy proteins essential for neuronal survival. Protection was dose-dependent with EC50 3.0 mM and extended to 64 h in K+-serum deprived apoptotic media. Neuronal extracts contain a spectrum of proximal (-2, -8, -9) and distal (-3, -6) caspases sensitive to Li+ on assay with preferred peptide substrates and by immunobloting. The lack of direct effect on activated cytosols indicates Li+ acts upstream only on intact cells, at sites for recruitment of pivotal procaspases. Alterations of procaspase-9 p46 and membrane-bound cytochrome c (Apaf-1) point to interaction with an intrinsic Mt-mediated pathway as one of the targets. The opposite effects on caspases and viability of immature or embryological neurons point to existence of alternative pathways that alter during neurite outgrowth suggesting the use of Li+ as a probe to unravel events relevant to neurogenesis.
Similar content being viewed by others
REFERENCES
Manji, H. K., Moore, G. J., and Chen, G. 1999. Lithium at 50: have the neuroprotective effects of this unique cation been overlooked? Biol. Psychiatry 46:929–940.
Volonte, C., Ciotti, M. T., and Merlo, D. 1994. LiCl promotes survival of GABAergic neurons from cerebellum and cerebral cortex: LiCl induces survival of GABAergic neurons. Neurosci. Lett. 172:6–10.
Volonte, C. and Rukenstein, A. 1993. Lithium chloride promotes short-term survival of PC12 cells after serum and NGFdeprivation. Lithium 4:211–219.
D'Mello, S. R., Anelli, R., and Calissano, P. 1994. Lithium induces apoptosis in immature cerebellar granule cells but promotes survival of mature neurons. Exp. Cell Res. 211:332–338.
Chen, R. W. and Chuang, D. M. 1999. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression. A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 274:6039–6042.
Green, D. R. and Reed, J. C. 1998. Mitochondria and apoptosis. Science 281:1309–1312.
Marks, N., Prasad, V., Berg, M. J., Green, M. R., and Saito, M. 1998. Li+ alters levels of caspase-3 in cerebellar neurons dependent on their differentiation. J. Neurochem. 71 (Supplement):S64.
Oberto, A., Marks, N., Evans, H. L., and Guidotti, A. 1996. Lead (Pb+2) promotes apoptosis in newborn rat cerebellar neurons: pathological implications. J. Pharmacol. Exp. Therapeut. 279:435–442.
Armstrong, R. C., Aja, T. J., Hoang, K. D., Gaur, S., Bai, X., Alnemri, E. S., Litwack, G., Karanewsky, D. S., Fritz, L. C., and Tomaselli, K. J. 1997. Activation of the CED3/ICE-related protease CPP32 in cerebellar granule neurons undergoing apoptosis but not necrosis. J. Neurosci. 17:553–562.
Eldadah, B. A., Yakovlev, A. G., and Faden, A. I. 1997. The role of CED-3-related cysteine proteases in apoptosis of cerebellar granule cells. J. Neurosci. 17:6105–6113.
Marks, N., Berg, M. J., Saito, M., and Guidotti, A. 1998. Activation of caspase-3 and apoptosis in cerebellar granule cells. J. Neurosci. Res. 52:334–341.
Kharlamov, E., Cagnoli, C. M., Atabay, C., Ikonomovic, S., Grayson, D. R., and Manev, H. 1995. Opposite effects of protein synthesis inhibitors on potassium deficiency-induced apoptotic cell death in immature and mature neuronal cultures. J. Neurochem. 65:1395–1398.
Levick, V., Coffey, H., and D'Mello, S. R. 1995. Opposing effects of thapsigargin on the survival of developing cerebellar granule neurons in culture. Brain Res. 676:325–335.
Cheema, Z. F., Wade, S. B., Sata, M., Walsh, K., Sohrabji, F., and Miranda, R. C. 1999. Fas/Apo [Apoptosis]-1 and associated proteins in the differentiating cerebral cortex: induction of caspasedependent cell death and activation of NF-kappaB. J. Neurosci. 19:1754–1770.
Raoul, C., Henderson, C. E., and Pettmann, B. 1999. Programmed cell death of embryonic motoneurons triggered through the Fas death receptor. J. Cell Biol. 147:1049–1062.
Felderhoff-Mueser, U., Taylor, D. L., Greenwood, K., Kozma, M., Stibenz, D., Joashi, U. C., Edwards, A. D., and Mehmet, H. 2000. Fas/CD95/APO-1 can function as a death receptor for neuronal cells in vitro and in vivo and is upregulated following cerebral hypoxic-ischemic injury to the developing rat brain. Brain Pathol. 10:17–29.
Brewer, G. J. 1995. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 42:674–683.
Thornberry, N. A., Rano, T. A., Peterson, E. P., Rasper, D. M., Timkey, T., Garcia-Calvo, M., Houtzager, V. M., Nordstrom, P. A., Roy, S., Vaillancourt, J. P., Chapman, K. T., and Nicholson, D. W. 1997. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272:17907–17911.
Talanian, R. V., Quinlan, C., Trautz, S., Hackett, M. C., Mankovich, J. A., Banach, D., Ghayur, T., Brady, K. D., and Wong, W. W. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272:9677–9682.
Smith, P. K., Krohn, R. I., Hermanson, G. T., Mallia, A. K., Gartner, F. H., Provenzano, M. D., Fujimoto, E. K., Goeke, N. M., Olson, B. J., and Klenk, D. C. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76–85.
Srinivasan, A., Roth, K. A., Sayers, R. O., Shindler, K. S., Wong, A. M., Fritz, L. C., and Tomaselli, K. J. 1998. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death. Differ. 5:1004–1016.
Nonaka, S., Katsube, N., and Chuang, D. M. 1998. Lithium protects rat cerebellar granule cells against apoptosis induced by anticonvulsants, phenytoin and carbamazepine. J. Pharmacol. Exp. Therapeut. 286:539–547.
Sun, X. M., MacFarlane, M., Zhuang, J., Wolf, B. B., Green, D. R., and Cohen, G. M. 1999. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. 274:5053–5060.
Yu, O. and Chuang, D. M. 1997. Neurotrophin protection against toxicity induced by low potassium and nitroprusside in cultured cerebellar granule neurons. J. Neurochem. 68:68–77.
Araki, T., Taniwaki, T., Becerra, S. P., Chader, G. J., and Schwartz, J. P. 1998. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J. Neurosci. Res. 53:7–15.
Cordeiro, M. L., Umbach, J. A., and Gundersen, C. B. 2000. Lithium ions Up-regulate mRNAs encoding dense-core vesicle proteins in nerve growth factor-differentiated PC12 cells. J. Neurochem. 75:2622–2625.
DeGregorio-Rocasolano, N., Gasull, T., and Trullas, R. 2001. Overexpression of neuronal pentraxin 1 is involved n neuronal death by low K+ cerebellar granule cells. J. Biol. Chem. 276:796–803.
Li, P., Nijhawan, D., Budihardjo, I., Srinivasula, S. M., Ahmad, M., Alnemri, E. S., and Wang, X. 1997. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91:479–489.
Keynes, R. D. and Swan, R. C. 1959. The permeability of frog muscle fibres to lithium ions. J. Physiol. 147:626–638.
Brenner, C. and Kroemer, G. 2000. Apoptosis. Mitochondria- the death signal integrators. Science 289:1150–1151.
Chai, J., Du, C., Wu, J. W., Kyin, S., Wang, X., and Shi, Y. 2000. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855–862.
Susin, S. A., Daugas, E., Ravagnan, L., Samejima, K., Zamzami, N., Loeffler, M., Costantini, P., Ferri, K. F., Irinopoulou, T., Prevost, M. C., Brothers, G., Mak, T. W., Penninger, J., Earnshaw, W. C., and Kroemer, G. 2000. Two Distinct Pathways Leading to Nuclear Apoptosis. J. Exp. Med. 192:571–580.
Li, H., Kolluri, S. K., Gu, J., Dawson, M. I., Cao, X., Hobbs, P. D., Lin, B., Chen, G., Lu, J., Lin, F., Xie, Z., Fontana, J. A., Reed, J. C., and Zhang, X. 2000. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 289:1159–1164.
Schuler, M., Bossy-Wetzel, E., Goldstein, J. C., Fitzgerald, P., and Green, D. R. 2000. p53 induces apoptosis by caspase activation through mitochondrial cytochrome c release. J. Biol. Chem. 275:7337–7342.
Moran, J., Itoh, T., Reddy, U. R., Chen, M., Alnemri, E. S., and Pleasure, D. 1999. Caspase-3 expression by cerebellar granule neurons is regulated by calcium and cyclic AMP. J. Neurochem. 73:568–577.
Yao, C. J., Lin, C. W., and Lin-Shiau, S. Y. 1999. Roles of thapsigargin-sensitive Ca2+ stores in the survival of developing cultured neurons. J. Neurochem. 73:457–465.
Yao, C. J., Lin, C. W., and Lin-Shiau, S. Y. 1999. Altered intracellular calcium level in association with neuronal death induced by lithium chloride. J. Fosmos. Med. Assoc. 98:820–826.
Zhu, L. P., Yu, X. D., Ling, S., Brown, R. A., and Kuo, T. H. 2000. Mitochondrial Ca(2+)homeostasis in the regulation of apoptotic and necrotic cell deaths. Cell Calcium 28:107–117.
Cooper, T. B. 1987. Pharmacokinetics of lithium. Pages 1365–1375, in Meltzer, H. Y. (eds.), Psychopharmacology: the third generation of progress, New York, N.Y.
Riedl, U., Barocka, A., Kolem, H., Demling, J., Kaschka, W. P., Schelp, R., Stemmler, M., and Ebert, D. 1997. Duration of lithium treatment and brain lithium concentration in patients with unipolar and schizoaffective disorder-a study with magnetic resonance spectroscopy. Biol. Psychiatry 41:844–850.
Kabakov, A. Y., Karkanias, N. B., Lenox, R. H., and Papke, R. L. 1998. Synapse-specific accumulation of lithium in intracellular microdomains: a model for uncoupling coincidence detection in the brain. Synapse 28:271–279.
Schulz, J. B., Weller, M., and Klockgether, T. 1996. Potassium deprivation-induced apoptosis of cerebellar granule neurons: a sequential requirement for new mRNA and protein synthesis, ICE-like protease activity, and reactive oxygen species. J. Neurosci. 16:4696–4706.
Yu, S. P., Yeh, C.-H., Sensi, S. L., Gwag, B. J., Canzoniero, L. M. T., Farhangrazi, Z. S., Ying, H. S., Tian, M., Dugan, L. L., and Choi, D. W. 1997. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 278:114–117.
Li, R. and El Mallahk, R. S. 2000. A novel evidence of different mechanisms of lithium and valproate neuroprotective action on human SY5Y neuroblastoma cells: caspase-3 dependency. Neurosci. Lett. 294:147–150.
Nicholson, D. W., Ali, A., Thornberry, N. A., Vaillancourt, J. P., Ding, C. K., Gallant, M, Gareau, Y., Griffin, P. R., Labelle, M., Lazebnik, Y. A., and et al. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376:37–43.
Hara, H., Friedlander, R. M., Gagliardini, V., Ayata, C., Fink, K., Huang, Z., Shimizu-Sasamata, M., Yuan, J., and Moskowitz, M. A. 1997. Inhibition of interleukin 1beta converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. U. S. A. 94:2007–2012.
Garcia-Calvo, M., Peterson, E. P., Leiting, B., Ruel, R., Nicholson, D. W., and Thornberry, N. A. 1998. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273:32608–32613.
D'Mello, S. R., Aglieco, F., Roberts, M. R., Borodezt, K., and Haycock, J. W. 1998. A DEVD-inhibited caspase other than CPP32 is involved in the commitment of cerebellar granule neurons to apoptosis induced by K+ deprivation. J. Neurochem. 70:1809–1818.
Marks, N. and Berg, M. J. 1999. Recent advances in neuronal caspases in development and neurodegeneration. Neurochem. Int. 35:195–220.
Janicke, R. U., Spengart, M. L., Wati, M. R., and Porter, A. G. 1998. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J. Biol. Chem. 273:9357–9360.
D'Mello, S. R., Kuan, C. Y., Flavell, R. A., and Rakic, P. 2000. Caspase-3 is required for apoptosis-associated DNA fragmentation but not for cell death in neurons deprived of potassium. J. Neurosci. Res. 59:24–31.
Lazebnik, Y. A., Kaufmann, S. H., Desnoyers, S., Poirier, G. G., and Earnshaw, W. C. 1994. Cleavage of poly(ADPribose) polymerase by a proteinase with properties like ICE. Nature 371:346–347.
Enari, M., Sakahira, H., Yokoyama, H., Okawa, K., Iwamatsu, A., and Nagata, S. 1998. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391:43–50.
Alvarez, G., Munoz-Montano, J. R., Satrustegui, J., Avila, J., Bogonez, E., and Diaz-Nido, J. 1999. Lithium protects cultured neurons against beta-amyloid-induced neurodegeneration. FEBS Lett. 453:260–264.
Lovestone, S., Davis, D. R., Webster, M. T., Kaech, S., Brion, J. P., Matus, A., and Anderton, B. H. 1999. Lithium reduces tau phosphorylation: effects in living cells and in neurons at therapeutic concentrations. Biol. Psychiatry 45:995–1003.
Wei, H., Leeds, P. R., Qian, Y., Wei, W., Chen, R., and Chuang, D. 2000. beta-Amyloid peptide-induced death of PC 12 cells and cerebellar granule cell neurons is inhibited by long-term lithium treatment. Eur. J. Pharmacol. 392:117–123.
Manji, H. K., Moore, G. J., and Chen, G. 2000. Lithium upregulates the cytoprotective protein Bcl-2 in the CNS in vivo: a role for neurotrophic and neuroprotective effects in manic depressive illness. J. Clin. Psychiatry 61 Suppl 9:82–96.
Moore, G. J., Bebchuk, J. M., Wilds, I. B., Chen, G., and Manji, H. K. 2000. Lithium-induced increase in human brain grey matter. Lancet 356:1241–1242.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marks, N., Saito, M., Green, M. et al. Opposite Effects of Lithium on Proximal and Distal Caspases of Immature and Mature Primary Neurons Correlate with Earlier Paradoxical Actions on Viability. Neurochem Res 26, 1311–1320 (2001). https://doi.org/10.1023/A:1014249517926
Issue Date:
DOI: https://doi.org/10.1023/A:1014249517926