Abstract
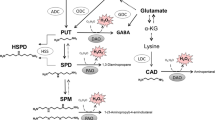
The aromatic amine, β-phenethylamine, was identified in various field-grown leguminous plants by analyses with HPLC, GC, GC-MS and 1H-NMR. High concentration of β-phenethylamine was generally detected only in mature root nodules, but not in other plant organs such as root, stem, leaf, pod and grain. Occurrence was specific to the root nodules formed by Bradyrhizobium infection. Ten of eleven legume crops including soybean [Glycine max (L.) Merr.], pigeon pea [Cajanus cajan (L.) Millsp.], adzuki bean (Vigna angularis), mung bean [V. radiata (L.) Wilczek] and cowpea (V. unguiculata) contained this aromatic amine, but groundnut (Arachis hypogaea L.) also nodulated by Bradyrhizobium sp. did not. Root nodules collected from garden pea (Pisum sativum L.), broad bean (Vicia fava L.), kidney bean (Phaseolus vulgaris L.) and various other herbaceous legumes nodulated by Rhizobium sp., Mesorhizobium sp., Sinorhizobium sp. or Azorhizobium caulinodans, and root-nodulated, woody non-legumes, nodulated by Frankia spp., contained little β-phenethylamine.
The amount of β-phenethylamine in Bradyrhizobium-infected nodules varied with the legume species and their cultivars, and most significantly, with nodule age. In field-grown soybean plants, nodule β-phenethylamine attained maximum concentration at the flowering stage and far exceeded that of the major polyamines of soybean nodules, putrescine and spermidine.
Similar content being viewed by others
References
Arora N 1954 Morphological development of the root and stem nodules of Aeschynomene indica L. Phytomorphology 4, 265–272.
Atkins C A 1991 Ammonia assimilation and export of nitrogen from the legume nodule. In Biology and Biochemistry of Nitrogen Fixation. Eds. M Dilworth and A Glenn. pp 293–319. Elsevier Science Publishers B.V., Amsterdam.
Bauer W D 1981 Infection of legumes by rhizobia. Annu. Rev. Plant Physiol. 32, 407–449.
Bergersen F J 1971 Biochemistry of symbiotic nitrogen fixation in legumes. Annu. Rev. Plant Physiol. 22, 121–140.
Carlson R W, Price N P J and Stacey G 1994 The biosynthesis of rhizobial lipo-oligosaccharide nodulation signal molecules. Mol. Plant-Microbe. Interact. 7, 684–695.
Chamber M A and Iruthayathas E E 1988 Nodulation and nitrogen fixation by fast-and slow-growing rhizobia strains of soybean on several temperate and tropical legumes. Plant Soil 112, 239–245.
DeLuca V 2000 Metabolic engineering of crops with the tryptophan decarboxylase of Catharanthus roseus. In Metabolic Engineering of Plant Secondary Metabolism. Eds. R Verpoorte and A W Alfermann. pp 179–194. Kluwer Academic Publishers, Dordrecht.
Delves A C, Mathews A, Day D A, Carter A S, Carrol B J and Gresshoff P M 1986 Regulation of the Rhizobium-soybean symbiosis by shoot and root factors. Plant Physiol. 82, 588–590.
Dreyfus B and Dommergues Y 1981 Nitrogen-fixing nodules induced by Rhizobium on the stem of the tropical legume Sesbania rostrata. FEMS Microbiol. Lett. 10, 313–317.
Dreyfus B, Garcia J L and Gillis M 1988 Characterization of Azorhizobium caulinodans gen. nov., a stem-nodulating nitrogen-fixing bacterium isolated from Sesbania rostrata. Int. J. Syst. Bacteriol. 38, 89–98.
Fitzgerald J S 1964 Alkaloids of the Australian Leguminosae. III. The occurrence of phenethylamine derivatives in Acacia species. Aust. J. Chem. 17, 160–162.
Flores H E and Galston A W 1982 Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol. 69, 701–706.
Flores H E and Martin-Tanguy J 1991 Polyamines and plant secondary metabolites. In Biochemistry ahd Physiology of Polyamines in Plants. Eds. R D Slocum and H E Flores. pp 57–76. CRC Press, Florida.
Fujihara S, Nakashima T and Kurogochi Y 1982 Occurrence of a new polyamine, canavalmine, in the sword bean Canavalia gladiata. Biochem. Biophys. Res. Commun. 107, 403–410.
Fujihara S and Harada Y 1989 Fast-growing root nodule bacteria produce a novel polyamine, aminobutylhomospermidine. Biochem. Biophys. Res. Commun. 165, 659–666.
Fujihara S and Yoneyama T 1993 Effects of pH and osmotic stress on cellular polyamine contents in the soybean rhizobia Rhizobium fredii P220 and Bradyrhizobium japonicum A1017. Appl. Environ. Microbiol. 59, 1104–1109.
Fujihara S, Abe H, Minakawa Y, Akao S and Yoneyama T 1994 Polyamines in nodules from various plant-microbe symbiotic associations. Plant Cell Physiol. 35, 1127–1134.
Galston A W and Kaur-Sawhney R 1990 Polyamines in Plant Physiology. Plant Physiol. 94, 406–410.
Ghosal S and Banerjee P K 1969 Alkaloids of the roots of Desmodium gangeticum. Aust. J. Chem. 22, 2029–2031.
Ghosal S, Mazumder U K and Mehta R 1972 Indole bases of Desmodium gyrans. Phytochemistry 11, 1863–1864.
Ghosal S and Srivastava R S 1973 Chemical investigation of Alhagi pseudalhagi (Bieb.) Desv.: ?-phenethylamine and tetrahydroisoquinoline alkaloids. J. Pharm. Sci. 62, 1555–1556.
Ghosal S, Srivastava R S, Banerjee P K and Dutta S K 1971 Alkaloids of Desmodium triflorum. Phytochemistry 10, 3312–3313.
Ghosal S, Srivastava R S, Bhattacharya S K and Debnath P K 1974 The active principles of Alhagi pseudalhagi: ?-phenethylamine and tetrahydroisoquinoline bases. Planta Med. 26, 318–326.
Halverson L J and Stacey G 1986 Signal exchange in plant microbe interactions. Microbiol. Rev. 50, 193–225.
Hamana K, Hamana H, Niitsu M, Samejima K, Sakane T and Yokota A 1994 Occurrence of tertiary and quaternary branched polyamines in thermophilic archaebacteria. Microbios. 79, 109–119.
Jordan D C 1984 Family III. Rhizobiaceae. In Bergey's Manual of Systematic Bacteriology. Eds. N R Kreig and J G Holt. pp 234–244. Williams & Wilkins, Baltimore.
Keller W J, McLaughlin J L and Brady L R 1973 Cactus alkaloids XV: ?-Phenethylamine derivatives from Coryphantha macromeris var. run runyonii. J. Pharm. Sci. 62, 408–411.
Martínez-Romero E, Caballero-Mellado J, Gándara B, Rogel M A, López Merino A, Wang E T, Fuentes-Ramírez L E, Toledo I, Martínez L, Hernández-Lucas I and Martínez-Romero J 2000 Ecological, phylogenetic and taxonomic remarks on diazotrophs and related genera. In Nitrogen Fixation: From Molecules to Crop Productivity. Eds. F O Pedrosa, M Hungria, M G Yates and W E Newton. pp 155–160. Kluwer Academic Publishers, Dordrecht.
Miller M S and Pepper I L 1988 Survival of a fast-growing strain of lupin rhizobia in sonoran desert soils. Soil Biol. Biochem, 20, 323–327.
Moro G A, Graziano M N and Coussio J D 1975 Alkaloids of Prosopis nigra. Phytochemistry 14, 827.
Norris D O 1965 Acid production by Rhizobium.: a unifying concept. Plant Soil 22, 143–166.
Okada M, Kawashima S and Imahori K 1979 Substrate binding characteristics of the active site of spermidine dehydrogenase from Serratia marcescens. J. Biochem. 85, 1235–1243.
Pate J S 1980 Transport and partitioning of nitrogenous solutes. Annu. Rev. Plant Physiol. 31, 312–340.
Peoples M B, Atkins C A, Pate J S, Chong K, Faizah A W, Suratmini P, Nurhayati D P, Bagnall D J and Bergersen F J 1991 Re-evaluation of the role of ureides in the xylem transport of nitrogen in Arachis species. Physiol. Plant. 83, 560–567.
Phillips G C and Kuehn G D 1991 Uncommon polyamines in plants and other organisms. In Biochemistry and Physiology of Polyamines in Plants. Eds. R D Slocum and H E Flores. pp 121–136. CRC Press, Florida.
Rolfe B G and Gresshoff P M 1988 Genetic analysis of legume nodule initiation. Annu. Rev. Plant Physiol. 39, 297–319.
Sadowsky M J, Keyser H H and Bohlool B B 1983 Biochemical characterization of fast-and slow-growing rhizobia that nodulate soybeans. Int. J. Syst. Bacteriol. 33, 716–722.
Stowers M D and Eaglesham A R J 1984 Physiological and symbiotic characteristics of fast-growing Rhizobium japonicum. Plant Soil 77, 3–14.
Streeter J G 1985 Nitrate inhibition of nodule growth and activity. Long term studies with a continuous supply of nitrate. Plant Physiol. 77, 321–324.
Trinick M J 1979 Structure of nitrogen-fixing nodules formed by Rhizobium on roots of Parasponia andersonii Planch. Can. J. Microbiol. 25, 565–578.
Trinick M J and Hadobas P A 1990 Symbiotic effectiveness of Bradyrhizobium strains isolated from Parasponia and tropical legumes on Parasponia host species. Plant Soil 124, 117–126.
Verpoorte R 2000 Secondary metabolism. In Metabolic Engineering of Plant Secondary Metabolism. Eds. R Verpoorte and A W Alfermann. pp 1–29. Kluwer Academic Publishers, Dordrecht.
Walden R, Cordeiro A and Tiburcio A F 1997 Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol. 113, 1009–1013.
Yelton M M, Yang S S, Edie S A and Lim S T 1983 Characterization of an effective salt-tolerant, fast-growing strain of Rhizobium japonicum. J. Gen. Microbiol. 129, 1537–1547.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fujihara, S., Terakado, J., Takenaka, M. et al. Specific occurrence of β-phenethylamine in root nodules formed from Bradyrhizobium-legume symbiosis. Plant and Soil 238, 123–132 (2002). https://doi.org/10.1023/A:1014232305742
Issue Date:
DOI: https://doi.org/10.1023/A:1014232305742