Abstract
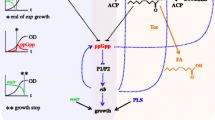
For evaluating the physiological status of cells, astringent response network was used. Fluorescence from intact E. coli, which has a plasmid encoding the green fluorescence protein (GFP) under the regulation of rpoS promoter, was monitored. Comparison of the response of different E. coli strains demonstrated an essential role of ppGpp in the expression of GFP, as it activated the rpoS promoter. The physiological status of intact cells, that depends on ppGpp accumulation in response to the nutritional status such as amino acid starvation, could therefore be monitored by measuring fluorescent intensity using this reporter gene.
Similar content being viewed by others
References
Cashel M (1969) The control of ribonucleic acid synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J. Biol. Chem. 244: 3133-3141.
Cashel M, Gentry DR, Hernandez VJ, Vinella D (1996) The stringent response. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, eds. Escherichia coli and Salmonella. Cellular and Molecular Biology, 2nd edn. Washington D.C.: American Society for Microbiology, pp. 1458-1496.
Gill RT, Valdes JJ, Bentley WE (2000) A comparative study of global stress gene regulation in response to overexpression of recombinant proteins in Escherichia coli. Metab. Eng. 2: 178-189.
Hengge-Aronis R (1996) Regulation of gene expression during entry into stationary phase. In: Neidhardt FC, Curtiss III R, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE, eds. Escherichia coli and Salmonella. Cellular and Molecular Biology, 2nd edn. Washington D.C.: American Society for Microbiology, pp. 1497-1512.
Jishage M, Ishihama A (1995) Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of sigma 70 and sigma 38. J. Bacteriol. 177: 6832-6835.
Jishage M, Iwata A, Ueda S, Ishihama A (1996) Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of four species of sigma subunit under various growth conditions. J. Bacteriol. 178: 5447-5451.
Lange R, Fischer D, Hengge-Aronis R (1995) Identification of transcriptional start sites and the role of ppGpp in the expression of rpoS, the structural gene for the sigma S subunit of RNA polymerase in Escherichia coli. J. Bacteriol. 177: 4676-4680.
Loewen PC, Hu B, Strutinsky J, Sparling R (1998) Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44: 707-717.
McCann MP, Kidwell JP, Matin A (1991) The putative sigma factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J. Bacteriol. 173: 4188-4194.
Neubauer P, Ahman M, Tornkvist M, Larsson G, Enfors SO (1995) Response of guanosine tetraphosphate to glucose fluctuations in fed-batch cultivations of Escherichia coli. J. Biotechnol. 43: 195-204.
Nyström T (1998) To be or not to be: the ultimate decision of the growth arrested bacterial cell. FEMS Microbiol. Rev. 21: 283-290.
Pizer LI, Merlie JP (1973) Effect of serine hydroxamate on phospholipid synthesis in Escherichia coli. J. Bacteriol. 114: 980-987.
Sambrook J, Fritsch EF, Hecker M (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
Schuster KC (2000) Monitoring the physiological status in bioprocesses on the cellular level. Adv. Biochem. Eng. Biotechnol. 66: 185-208.
Schweder T, Kruger E, Xu B, Jurgen B, Blomsten G, Enfors SO, Hecker M (1999) Monitoring of genes that respond to processrelated stress in large-scale bioprocesses. Biotechnol. Bioeng. 65: 151-159.
Tanaka K, Handel K, Loewen PC, Takahashi H (1997) Identification and analysis of the rpoS-dependent promoter of katE, encoding catalase HPII in Escherichia coli. Biochim. Biophys. Acta 1352: 161-166.
Teich A, Meyer S, Lin HY, Andersson L, Enfors S, Neubauer P (1999) Growth rate related concentration changes of the starvation response regulators sigmaS and ppGpp in glucose-limited fed-batch and continuous cultures of Escherichia coli. Biotechnol. Prog. 15: 123-129.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Funabashi, H., Mie, M., Yanagida, Y. et al. Fluorescent monitoring of cellular physiological status depending on the accumulation of ppGpp. Biotechnology Letters 24, 269–273 (2002). https://doi.org/10.1023/A:1014032711683
Issue Date:
DOI: https://doi.org/10.1023/A:1014032711683