Abstract
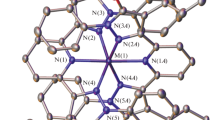
Complexes of CrIII, MnII, FeIII, CoII, NiII and CuII containing a macrocyclic pentadentate nitrogen–sulphur donor ligand have been prepared via reaction of a pentadentate ligand (N3S2) with transition metal ions. The N3S2 ligand was prepared by [1 + 1] condensation of 2,6-diacetylpyridine with 1,2-di(o-aminophenylthio(ethane. The structures of the complexes have been elucidated by elemental analyses, molar conductance, magnetic susceptibility measurements, i.r., electronic and e.p.r. spectral studies. The complexes are of the high spin type and are six-coordinate.
Similar content being viewed by others
References
D.E. Fenton, in A.G. Sykes (Ed), Advances in Inorganic and Bioinorganic Mechanism, Academic Press, London, 1983, Vol. 2, p. 187.
K.D. Karlin and J. Zubieta, Copper Coordination Chemistry and Biochemistry: Biochemical and Inorganic Perspectives, Adenine press, Guilderland, NY 1983.
K.D. Karlin and J. Zubieta, Biological and Inorganic Copper Chemistry, Vols. 1 and 2, Adenine Press, Guilderland, NY 1986.
D.E. Fenton, U. Casellato, P.A. Vigato and M. Vidali, Inorg. Chim. Acta, 62, 57 (1982).
P. Guerriero, P.A. Vigato, D.E. Fenton and P.C. Hellier, Acta Chem. Scand., 46, 1025 (1992).
R.D. Willet, D. Gatteschi and O. Kahn, Magneto-Structural Correlation in Exchange Coupled Systems, Nato ASI series, Reidel, Dordrecht, 1983.
U. Casellato, P.A. Vigato, D.E. Fenton and M. Vidali, Chem. Soc. Rev., 8, 199 (1979).
S.E. Groh, Isr. J. Chem., 15, 277 (1976/1977).
F.L. Urbach, in H. Sigel (Ed), Metal Ions in Biological Systems, No. 13, Copper proteins, Dekker, Basel, 1981, p. 73, and refs. cited therein.
P.K. Coughlin and S.J. Lippard, J. Am. Chem. Soc., 106, 2328 (1984); Y. Agnus, R. Louis, J.P. Gisselbrechi and R. Weiss, J. Am. Chem. Soc., 106, 93 (1984); D. Lussac, J.M. Savariauli, P.C. Cassou and J.P. Tuchangues, J. Chem. Soc., Dalton Trans., 1225 (1988); H. Dril, H.R. Chang, X. Zhang, S.K. Larsen, J.A. Polenza, C.G. Pierpont, H.J. Schugar, S.S. Isted and D.N. Hendrickson, J. Am. Chem. Soc., 109, 6207 (1987).
T.M. Garrett, J. Mc Murry, M.W. Hosseini, Z.E. Reyes, F.E. Hahn and K.N. Raymonds, J. Am. Chem. Soc., 113, 2965 (1991).
G. De Santis, L. Fabbrizzil, M. Licchelli, C. Mangano, P. Pallaviani and A. Poggi, Inorg. Chem., 32, 854 (1993).
R.D. Hancock, G. Pattrick, P.W. Wade and G.D. Hosken, Pure Appl. Chem., 65, 473 (1993).
R.L. Webb, M.L. Mino, E.L. Blinn and A.A. Pinkerton, Inorg. Chem., 32, 1396 (1993).
R.D. Cannon, B. Chiswell and L.M. Venanzi, J. Chem. Soc(A)., 1277 (1967).
S.C. Cummingsand D.H. Busch, J. Am. Chem. Soc., 92, 1924 (1970).
N.S. Gill, R.H. Nuttall and D.E. Scaife, J. Inorg. Nucl. Chem., 18, 79 (1961).
B.N. Figgis, Introduction to Ligand Field Theory, Wiley, New York, 1978.
C. Preti and G. Tosi, Aust. J. Chem., 20, 543 (1976).
J.G. Muller, X. Chen, A.C. Dadiz, S.E. Rohita and C.S. Burrows, Pure Appl. Chem., 65, 545 (1993).
V.B. Rana, D.P. Singh, P. Singh and M. Pteotia, Transition Met. Chem., 6, 36 (1981).
A.B.P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1968.
J.C. Donini, B.R. Hallestone and A.B.P. Lever, Inorg. Chem., 22, 225 (1977).
A. Earnshaw, L.F. Larkworthy and K.C. Patel, J. Chem. Soc(A)., 1339 (1969).
I.M. Procter, R.J. Hathaway and P. Nicholls, J. Chem. Soc(A)., 1678 (1968); (b) A.A.G. Tomlinson, R.J. Hathaway, D.E. Billing and P. Nicholls, J. Chem. Soc(A)., 65 (1969); S.N. Choi, E.R. Menzil and J.R. Waison, J. Inorg. Nucl. Chem., 39, 417 (1977); (c) K.G. Kocwin and W. Wojciechowski, Transition Met. Chem., 21, 312 (1996); (d) S.K. Ha.mann and J. Goster, J. Solid State Chem., 44, 343 (1982).
F.K. Kneubuhl, J. Chem. Phys., 33, 1074 (1960).
B.J. Hathaway and D.E. Billing, Coord. Chem. Rev., 6, 143 (1970).
B.J. Hathaway, in J.N. Bradley and R.D. Gillard (Eds), Essays in Chemistry, Academic Press, New York, 1971, p. 61.
B.J. Hathaway, R.J. Dudley and P. Nicholls, J. Chem. Soc(A)., 1845 (1968).
R.J. Dudley and B.J. Hathaway, J. Chem. Soc(A)., 1725 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chandra, S., Gupta, K. Chromium(III), manganese(II), iron(III), cobalt(II), nickel(II) and copper(II) complexes with a pentadentate, 15-membered new macrocyclic ligand. Transition Metal Chemistry 27, 196–199 (2002). https://doi.org/10.1023/A:1013935602736
Issue Date:
DOI: https://doi.org/10.1023/A:1013935602736