Abstract
Purpose. It was previously reported that co-administration of H-MAP to the airways of the lungs significantly influenced the absorption, disposition, and effect of insulin in a dose-dependent fashion. Doses of H-MAP (16 mg/kg) and insulin (1.3 U/kg) required to achieve maximum pharmacokinetic and pharmacodynamic responses were determined. The purpose of the present study was to evaluate the effects of insulin and H-MAP spray-instilled (SI) to rats on the physiology of the lung. A short-term, single-dose study of insulin alone and combined with H-MAP was performed.
Methods. Solutions of either insulin (INS), H-MAP, or insulin plus H-MAP (INMA) were SI to the lungs of rats. Lipopolysaccharide solution (LPS) and sodium dodecyl sulfate solution (SDS) were used as positive controls, and normal saline (SAL) was used as negative control. Animals were sacrificed at various time points and bronchoalveolar lavage (BAL) was conducted. BAL fluid was analyzed for local markers of lung injury, such as total cell numbers, differential cell count, total protein content and enzyme activities of lactate dehydrogenase (LDH), alkaline phosphatase (ALP) and N-acetyl glucosaminidase (NAG).
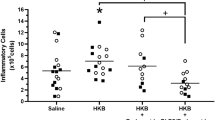
Results. SI of any solution, including normal saline, seems to have a minor but detectable effect on the normal physiology of the lung. SI of positive control solutions resulted in most markers of immunity and lung injury being significantly elevated, notably enzyme activity and white cell infiltrate. In contrast, SI of INS produced a response similar to that of SAL. SI of INMA resulted in a small transient response characterized by a slight increase in the proportion of neutrophils at 24 h, which decreased with time and was comparable to that of SAL at 72 h. SI of H-MAP resulted in a response similar to that from INMA; however an additional transient increase in LDH activity was noted which may be related to the mechanism of action of H-MAP.
Conclusion. SI of INS, H-MAP, and INMA caused no apparent toxicity at the doses studied. A small, transient effect of H-MAP was noted which did not elicit the full complement of inflammatory markers and which was substantially smaller than the effect of either LPS or SDS.
Similar content being viewed by others
REFERENCES
J. Yu and Y. W. Chien. Pulmonary drug delivery: Physiological and mechanistical aspects. Crit. Rev. Ther. Drug Carrier Syst. 14:395-453 (1997).
G. P. Carino and E. Mathiowitz. Oral insulin delivery. Adv. Drug Deliv. Rev. 35:249-257 (1999).
J. S. Patton, J. Bukar, and S. Nagarajan. Inhaled insulin. Adv. Drug Deliv. Rev. 35:235-247 (1999).
B. L. Laube, A. Georgopoulus, and G. K. Adams. Aerosolized insulin delivered trhough the lungs is effective in normalizing plasma glucose levels in non-insulin dependent diabetic subjects. J. Biopharm. Sci. 3:163-169 (1992).
I. Gonda, J. A. Schuster, R. M. Rubsamen, P. Lloyd, D. Cipolla, and S. J. Farr. Inhalation delivery systems with compliance and disease management capabilities. J. Control. Release 53:269-274 (1998).
F. M. Wigley, J. H. Londono, S. H. Wood, J. C. Ship, and R. H. Waldman. Insulin across respiratory mucosa by aerosol delivery. Diabetes 20:552-556 (1971).
R. B. Elliot, B. W. Edgar, C. C. Pilcher, C. Questad, and J. McMaster. Parenteral absorption of insulin from the lung in diabetic children. Aust. Paediatr. J. 23:293-297 (1987).
H. Yoshida, K. Okumura, R. Hori, T. Anmo, and H. Yamaguchi. Absorption of insulin delivered to rabbit trachea using aerosol dosage form. J. Pharm. Sci. 68:670-671 (1979).
P. Colthorpe, S. J. Farr, G. Taylor, I. J. Smith, and D. Wyatt. The pharmacokinetics of pulmonary-delivered insulin: A comparison of intratracheal and aerosol administration to the rabbit. Pharm. Res. 9:764-768 (1992).
F. Komada, S. Iwakawa, N. Yamamoto, N. Sakakibara, and K. Okumura. Intratracheal delivery of peptide and protein agents from solution and aerosol by rat lung. J. Pharm. Sci. 83:863-867 (1994).
S. Kobayashi, S. Kondo, and K. Juni. Study on pulmonary delivery of salmon calcitonin in rats: effect of protease inhibitors and absorption enhancers. Pharm. Res. 11:1239-1243 (1994).
A. Yamamoto, S. Umemori, and S. Muranishi. Absorption enhancement of intrapulmonary administered insulin by various absorption enhancers and protease inhibitors in rats. J. Pharm. Pharmacol. 46:14-18 (1994).
S. C. Raehs, J. Sandow, K. Wirth, and H. P. Merkle. The adjuvant effect of bacitracin on nasal absorption of gonadorelin and buserelin in rat. Pharm. Res. 5:689-693 (1988).
M. Saffran, C. Bedra, G. S. Kumar, and D. C. Neckers. Vasopressin: A model for the study of effects of additives on the oral and rectal administration of peptide drugs. J. Pharm. Sci. 77:33-38 (1988).
S. Suarez, D. O'Toole, J. Smart, and A. J. Hickey. The effect of an aromatic amido acid (B08) on the pharmacokinetics and pharmacodynamics of insulin delivered to the lungs of rats. AAPS Pharm Sci. Suppl. 1:2209 (1999).
S. J. Milstein, H. Leipold, D. Sarubbi, A. Leone-Bay, G. M. Mlynek, J. R. Robinson, M. Kasimova, and E. Freire. Partially unfolded proteins efficiently penetrate cell membranes — implications for oral drug delivery. J. Control. Release 53:259-267 (1998).
J. Linden and S. I. Rennard. Bronchoalveolar lavage. ASCP Press. Chicago, Illinois, 1988.
R. F. Henderson, J. M. Benson, F. F. Hahn, C. H. Hobbs, R. K. Jones, J. L. Mauderly, R. O. McClellan, and J.A. Pickrell. New approaches for the evaluation of pulmonary toxicity: Bronchoalveolar lavage fluid analysis. Fundam. Appl. Toxicol. 5:451-458 (1985).
H. Konzett and R. Roessler. Versuchsanordnung zu untersuchungen an der bronchialmuskulator, Naunyn Schmiedeberg. Arch. Exp. Pathol. Pharmakol. 195:71-74 (1940).
J. S. Franzone, R. Cirillo, and P. Biffignandi. Doxofylline exerts a prophylactic effect against bronchoconstriction and pleurisy induced by PAF. Eur. J. Pharmacol. 165:269-277 (1989).
SAS Institute Inc; SAS/STAT User's Guide, Version 6, 4th edition, Volume 2. The SAS Institute Inc., Cary, North Carolina 1989.
R. J. Pettis and A. J. Hickey. Aerosol delivery of peptide-immuno-modulators to rodent lungs. In J. P. Tam and P. T. P. Kaumaya (eds.), Peptides, Frontiers of Peptide Science. Kluwer Academic Publishers, Boston, Massachusetts 1999 pp. 845-846.
J. M. Antonini, G. G. Murthy, and J. D. Brain. Responses to welding fumes: Lung injury, inflammation and the release of tumor necrosis factor-alpha and interleukin-1 beta. Exp. Lung Res. 23:205-227 (1997).
A. Lopez and S. Yong. Injury vs. inflammatory response in the lungs of rats intratracheally inoculated with bacterial lipopolysaccharide. Am. J. Vet. Res. 47:1287-1292 (1986).
C. R. Behl, H. K. Pimplaskar, A. P. Sileno, W. J. Xia, W. J. Gries, J. C. deMeireles, and V. D. Romeo. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv. Drug Deliv. Rev. 29:117-133 (1998).
V. H. L. Lee. Protease inhibitors and penetration enhancers as approach to modify peptide absorption. J. Control Release 13:213-223 (1990).
V. H. L. Lee. Enzymatic barriers to peptide and protein absorption and the use of penetration enhancers to modify absorption. In: Delivery Systems for Peptide Drugs. Vol 125. S.S. Davies, Ed., Plenum Press, New York p. 87-104, (1986).
Subcommittee on Pulmonary Toxicology. Committee on Biologic Markers. Biologic Markers in Pulmonary Toxicology. National Academy Press. Washington D.C., 1989.
D. A. Edwards, J. Hanes, G. Caponetti, J. Hrkach, A. Ben-Jebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lothan, and R. Langer. Large porous particles for pulmonary drug delivery. Science 276: 1868-1871 (1997).
D. A. Edwards, A. Ben-Jebria, and R. Langer. Recent advances in pulmonary drug delivery using large, porous inhaled particles. J. Appl. Physiol. 84:379-385 (1998).
A. Leone-Bay, N. Santiago, D. Achan, K. Chaudhary, F. DeMorin, L. Falzarano, S. Haas, S. Kalbag, D. Kaplan, H. Leipold, C. Lercara, D. O'Toole, T. Rivera, C. Rosado, D. Sarubbi, E. Vuocolo, N. Wang, S. Milstein, and R.A. Baughman. N-acylated alpha-amino acids as novel oral delivery agents for proteins. J. Med. Chem. 38:4263-4269 (1995).
A. Leone-Bay, K. Ho, R. Agarwal, R. A. Baughman, K. Chaudhary, F. DeMorin, L. Genoble, C. Mc Innes, C. Lercara, S. Milstein, D. O'Toole, D. Sarubbi, D. Variano, and D.R. Paton. 4-[4-[(2-Hydroxybenzoyl)amino]phenyl]butyric acid as a novel oral delivery agent for recombinant human growth hormone. J. Med. Chem. 39:2571-2578 (1996).
G. Schatz and B. Dobberstein. Common principles of protein translocation across membranes. Science 271:1519-1526 (1996).
K. Verner and G. Schatz. Protein translocation across membranes. Science 241:1307-1313 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garcia-Contreras, L., Sarubbi, D., Flanders, E. et al. Immediate and Short-Term Cellular and Biochemical Responses to Pulmonary Single-Dose Studies of Insulin and H-MAP. Pharm Res 18, 1685–1693 (2001). https://doi.org/10.1023/A:1013314311619
Issue Date:
DOI: https://doi.org/10.1023/A:1013314311619