Abstract
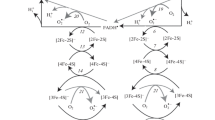
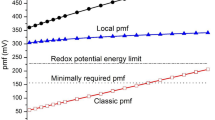
Direct nonenzymatic oxidation of semiquinone by oxygen is one of the main sources of superoxide radicals \(\left( {{O}_{2}^{\overline \cdot } } \right)\) in mitochondria. Using all the known data on hepatocyte mitochondria, we have revealed the correlation between the rate of superoxide generation by the bc 1complex and the transmembrane potential (ΔΨ). Assuming that the main electrogenic stage of the Qcycle is the electron transfer between the cytochrome bhemes, then the rate of superoxide generation sharply increases when ΔΨ grows from 150 to 180 mV. However, this interrelation is ambiguous. Indeed, the increase of the generation rate with the growth of the potential can occur faster when succinate dehydrogenase is inhibited by malonate than when external ADP is exhausted. When the potential is changed by adding phosphate or potassium (K+), the rate of \(\left( {{O}_{2}^{\overline \cdot } } \right)\) production remains constant, although the comparison of the rates at the same ΔΨ reveals the effect of phosphate or potassium. It turned out that the rate of \(\left( {{O}_{2}^{\overline \cdot } } \right)\) generation is a function of \(\Delta \overline {\mu } _{H}\) rather than any of its components. Phosphate and K+have practically no influence on \(\Delta \overline {\mu } _{H}\), since the change in ΔΨ is compensated by ΔpH. The rate of superoxide generation by the bc 1complex is a multiple function of the electron-transfer activity of enzymes, the processes determining the membrane potential (e.g., loading), and the oxygen concentration. The kinetic model proposed in this work may serve to understand how the superoxide production is regulated.
Similar content being viewed by others
References
Skulachev, V.P., Membrane Bioenergetics, Berlin: Springer, 1988.
Skulachev, V.P., Quart. Rev. Biophys., 1997, vol. 29, pp. 169–202.
Korshunov, S.S., Skulachev, V.P., and Starkov, A.A., FEBS Lett., 1997, vol. 416, pp. 15–18.
Boveris, A. and Chance, B., Biochem. J., 1973, vol. 134, pp. 707–716.
Hansford, R.G., Hogue, B.A., and Mildaziene, V., J. Bioenerg. Biomembr., 1997, vol. 29, pp. 89–95.
Korshunov, S.S., Korkina, O.V., Ruuge, E.K., Skulachev, V.P., and Starkov, A.A., FEBS Lett., 1998, vol. 435, pp. 215–218.
Bohnensack, R., J. Bioenerg. Biomemb., 1982, vol. 14, pp. 45–61.
Korzeniewski, B. and Froncisz, W., Biochim. Biophys. Acta., 1991, vol. 1060, pp. 210–223.
Korzeniewski, B., Biophys. Chemistry, 1996, vol. 57, pp. 143–153.
Korzeniewski, B., Mol. Cell. Biochem., 1998, vol. 184, pp. 345–358.
Van Dam, K., Westerhoff, H.V., Krab, K., Van der Meer, R., and Arents, J.C., Biochim. Biophys. Acta, 1980, vol. 591, pp. 240–250.
Demin, O.V., Kholodenko, B.N., and Skulachev, V.P., Mol. Cell. Biochem., 1998, vol. 184, pp. 21–33.
Drachev, L.A., Kaurov, B.S., Mamedov, M.D., Mulkidjanian, A.Y., Semenov, A.Y., Shinkarev, V.P., Skulachev, V.P., and Verkhovsky, M.I., Biochim. Biophys. Acta, 1989, vol. 973, pp. 189–197.
Semenov, A.Y., FEBS Lett., 1993, vol. 321, pp. 1–5.
Harris, E.J. and Bangham, J.A., J. Memb. Biol., 1972, vol. 9, pp. 141–154.
Alberty, R.A., J. Biol. Chem., 1969, vol. 244, pp. 3290–3302.
Lawson, J.W.R. and Veech, R.L., J. Biol. Chem., 1979, vol. 254, pp. 6528–6537.
Reich, J.G. and Rohde, K., Biomed. Bioch. Acta, 1983, vol. 42, pp. 37–46.
Demin, O.V., Vesterkhoff, Kh. V., and Kholodenko, B.N., Biokhimiya, 1998, vol. 63, pp. 37–53.
Boork, J. and Wennestrom, H., Biochim. Biophys. Acta, 1984, vol. 767, pp. 314–320.
Reynolds, I.A., Johnson, E.A., and Tanford, C., Proc. Natl. Acad. Sci. USA, 1985, vol. 82, pp. 6869–6873.
Liu, S.-s. and Huang, J.P., Molecular Mechanisms and Health Effects, Proc. Int. Symp. on Natural Antioxidants, Moores, D., Ed., Champaign: AOCS Press IL, 1996, pp. 513–529.
Liu, S.-s., Biosci. Reports., 1997, vol. 17, pp. 259–272.
Kunz, W., Bohnensack, R., Bohme, G., Kuster, U., Letko, G., and Schonfeld, P., Arch. Biochem. Biophys., 1981, vol. 209, pp. 219–229.
Letko, G., Kuster, U., Duszynski, J., and Kunz, W., Biochim. Biophys. Acta, 1980, vol. 593, pp. 196–203.
Kingsley, P.B. and Feigenson, G.W., Biochim. Biophys. Acta, 1981, vol. 635, pp. 602–618.
Trumpower, B.L., Biochim. Biophys. Acta, 1981, vol. 639, pp. 129–155.
Bowyer, J.R. and Trumpower, B.L., J. Biol. Chem., 1981, vol. 256, pp. 2245–2251.
Rich, P.R., Biochim. Biophys. Acta, 1984, vol. 768, pp. 53–79.
Wrigglesworth, J.M., Elsden, J., Chapman, A., der Water, N.V., and Grahn, M.F., Biochim. Biophys. Acta, 1988, vol. 936, pp. 452–464.
Jones, D., Am. J. Physiol., 1986, vol. 250, pp. C663–C675.
Green, D.E. and Wharton, D.C., Biochem. Z., 1963, vol. 336, pp. 335–346.
Srere, P.A., Trends Biol. Sci., 1981, vol. 6, pp. 4–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Demin, O.V., Goryanin, I.I., Kholodenko, B.N. et al. Kinetic Modeling of Energy Metabolism and Superoxide Generation in Hepatocyte Mitochondria. Molecular Biology 35, 940–949 (2001). https://doi.org/10.1023/A:1013211007465
Issue Date:
DOI: https://doi.org/10.1023/A:1013211007465