Abstract
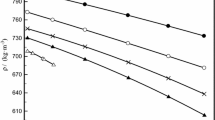
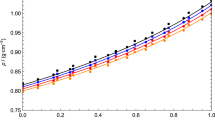
The dynamic viscosity η of the binary mixture 1-methylnaphthalene+2,2,4,4,6,8,8-heptamethylnonane was measured in the temperature range 293.15 to 353.15 K (in progressive 10 K steps) at pressures of 0.1, 20, 40, 60, 80, and 100 MPa. The composition of the system is described by nine molar fractions (0 to 1 in 0.125 progressive steps). The density ρ was measured at pressures from 0.1 to 60 MPa in progressive 5 MPa steps. The measurements of η are used to determine the excess viscosity η E and the excess activation energy of flow ΔG E as a function of pressure, temperature, and composition. Some models have been used to represent the viscosity of this binary mixture.
Similar content being viewed by others
REFERENCES
P. Daugé, A. Baylaucq, and C. Boned, High Temp. High Press. 31:665 (1999).
P. Daugé, X. Canet, A. Baylaucq, and C. Boned, High Temp. High Press. 33:213 (2001).
A. Et-Tahir, C. Boned, B. Lagourette, and P. Xans, Int. J. Thermophys. 16:1309 (1995).
M. Kanti, B. Lagourette, J. Alliez, and C. Boned, Fluid Phase Equil. 65:291 (1991).
U. G. Krahn and G. Luft, J. Chem. Eng. Data 39:670 (1994).
J. Zhang and H. Liu, J. Chem. Eng. Data 3:269 (1991).
F. Olive, K. R. Patil, A. Coronas, and F. Fernandez, Int. J. Thermophys. 15:661 (1994).
D. Papaioannou, M. Bridakis, and C. G. Panayiotou, J. Chem. Eng. Data 38:370 (1993).
L. Grunberg and A. H. Nissan, Nature 164:799 (1949).
P. K. Katti and M. M. Chaudhri, J. Chem. Eng. Data 9:442 (1964).
S. Glasstone, K. J. Laidler, and H. Eyring, The Theory of Rate Processes (McGraw–Hill, New York, 1941).
P. Cea, C. Lafuente, J. P. Morand, F. M. Royo, and J. S. Urieta, Phys. Chem. Liquids 29:69 (1995).
E. Heric and J. G. Brewer, J. Chem. Eng. Data 12:574 (1967).
I.L. Acevedo, M. A. Postigo, and M. Katz, Phys. Chem. Liquids 21:87 (1990).
R. Bravo, M. Pintos, A. Amigo, and M. Garcia, Phys. Chem. Liquids 22:245 (1991).
M. Moha-Ouchane, C. Boned, A. Allal, and M. Benseddik, Int. J. Thermophys. 19:161 (1998).
C. Boned, M. Moha-Ouchane, A. Allal, and M. Benseddik, Int. J. Thermophys. 19:1325(1998).
M. Kanti, H. Zhou, S. Ye, C. Boned, B. Lagourette, H. Saint-Guirons, P. Xans, and F. Montel, J. Phys. Chem. 93:3860 (1989).
J. H. Dymond and M. A. Awan, Int. J. Thermophys. 10:941 (1989).
M. J. Assael, J. H. Dymond, M. Papadaki, and P. M. Patterson, Fluid Phase Equil. 75:245 (1992).
A. Baylaucq, M. Moha-Ouchane, and C. Boned, Phys. Chem. Liquids 38:353 (2000).
M. J. Assael, J. H. Dymond, M. Papadaki, and P. M. Patterson, Int. J. Thermophys. 13:659 (1992).
H. Mensah-Brown and W.A. Wakeham, Int. J. Thermophys. 15:117 (1994).
A. Allal, M. Moha-Ouchane, and C. Boned, Phys. Chem. Liquids 39:1 (2001).
P. Daugé, Thèse de Doctorat (Université de Pau, Pau, France, 1999).
A. Allal, C. Boned, and P. Daugé, Phys. Chem. Liquids (in press).
S. E. Quiñones-Cisneros, C. K. Zéberg-Mikkelsen, and E. H. Stenby, Fluid Phase Equil. 169:249 (2000).
D.-Y. Peng and D. B. Robinson, Ind. Eng. Chem. Fund. 15:59 (1976).
S. E. Quiñones-Cisneros, C. K. Zéberg-Mikkelsen, and E. H. Stenby, Fluid Phase Equil. 178:1 (2001).
C. K. Zéberg-Mikkelsen, Ph.D. thesis (Technical University of Denmark, Lyngby, Denmark, 2001).
T. E. Daubert and R. P. Danner, Physical and Thermodynamic Properties of Pure Chemicals Data Compilation (Hemisphere, New York, 1989)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Canet, X., Daugé, P., Baylaucq, A. et al. Density and Viscosity of the 1-Methylnaphthalene+2,2,4,4,6,8,8-Heptamethylnonane System from 293.15 to 353.15 K at Pressures up to 100 MPa. International Journal of Thermophysics 22, 1669–1689 (2001). https://doi.org/10.1023/A:1013182715406
Issue Date:
DOI: https://doi.org/10.1023/A:1013182715406