Abstract
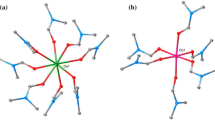
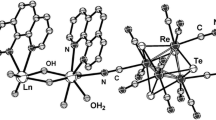
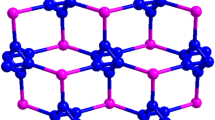
The compound [Mn(H2O)4]3[W4S4(CN)12]·nH2O (1) was prepared by the reaction of an aqueous solution of MnSO4 with a glycerol solution of KCs5[W4S4(CN)12]·4H2O. Complex 1 was characterized by X-ray diffraction analysis and IR spectroscopy. According to the X-ray diffraction data, the three-dimensional polymeric framework of 1 comprises only 24.2% of the unit-cell volume. The volume of the cavities per formula unit is 1664 Å3. The cavities are occupied by highly disordered water molecules.
Similar content being viewed by others
References
G. Ferey, J. Solid State Chem., 2000, 152, 37.
A. K. Cheetham, G. Ferey, and T. Loiseau, Angew. Chem., Int. Ed. Engl., 1995, 38, 3268.
H. Li, A. Laine, M. O'Keeffe, and O. M. Yaghi, Science, 1999, 283, 1145.
G. Y. Yang and S. C. Sevov, J. Am. Chem. Soc., 1999, 121, 8389.
D. Hagrman, P. J. Hagrman, and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 3165.
M. Wachhold, K. K. Rangan, M. Lei, M. F. Thorpe, S. J. L. Billinge, V. Petkov, J. Heising, and M. G. Kanatzidis, J. Solid State Chem., 2000, 152, 21.
M. Ishaque Khan, J. Solid State Chem., 2000, 152, 105.
S. Noro, S. Kitagawa, M. Kondo, and K. Seki, Angew. Chem., Int. Ed., 2000, 39, 2082.
K. R. Dunbar and R. A. Heintz, Prog. Inorg. Chem., 1997, 45, 283.
T. Iwamoto, Comprehensive Supramolecular Chemistry, Eds. J. L. Atwood, J. E. D. Davies, D. D. Macnicol, and F. Vögtle, Pergamon, 1996, 6, 643.
B. H. Chadwick and A. G. Sharpe, Adv. Inorg. Radiochem., 1966, 8, 83.
I. V. Tananaev, G. B. Seifer, Yu. Ya. Kharitonov, V. G. Kuznetsov, and A. P. Korol'kov, Khimiya ferrotsianidov [Chemistry of Ferrocyanides], Nauka, Moscow, 1971, 320 pp. (in Russian).
N. G. Naumov, A. V. Virovets, and V. E. Fedorov, Zh. Strukt. Khim., 2000, 41, 609 [Russ. J. Struct. Chem., 2000, 41, 499 (Engl. Transl.)].
N. G. Naumov, A. V. Virovets, M. N. Sokolov, S. B. Artemkina, and V. E. Fedorov, Angew. Chem. Int. Ed., 1998, 37, 1943.
Yu. V. Mironov, A. V. Virovets, S. B. Artemkina, and V. E. Fedorov, Angew. Chem., Int. Ed., 1998, 37, 2507.
N. G. Naumov, S. B. Artemkina, A. V. Virovets, and V. E. Fedorov, J. Solid State Chem., 2000, 153, 195.
N. G. Naumov, A. V. Virovets, and V. E. Fedorov, Inorg. Chem. Commun., 2000, 3, 71.
M. P. Shores, L. G. Beauvais, and J. R. Long, J. Am. Chem. Soc., 1999, 121, 775.
M. P. Shores, L. G. Beauvais, and J. R. Long, Inorg. Chem., 1999, 38, 1648.
V. P. Fedin, A. V. Virovets, I. V. Kalinina, V. N. Ikorskii, M. R. J. Elsegood, and W. Clegg, Eur. J. Inorg. Chem., 2000, 2341.
V. P. Fedin, I. V. Kalinina, D. G. Samsonenko, Y. V. Mironov, M. N. Sokolov, S. V. Tkachev, A. V. Virovets, N. V. Podberezskaya, M. R. J. Elsegood, W. Clegg, and A. G. Sykes, Inorg. Chem., 1999, 38, 1956.
N. G. Naumov, D. V. Soldatov, J. A. Ripmeester, S. B. Artemkina, and V. E. Fedorov, Chem. Commun., 2001, 571.
A. Goto, T. Hondoh, and S. Mae, J. Chem. Phys., 1990, 93, 1412.
L. G. Dowell and A. P. Rinfret, Nature, 1960, 188, 1144.
M. R. J. Elsegood, A. V. Virovets, D. G. Samsonenko, I. V. Kalinina, and V. P. Fedin, Zh. Strukt. Khim., 2000, 41, 1290 [Russ. J. Struct. Chem., 2000, 41 (Engl. Transl.)].
V. P. Fedin, D. G. Samsonenko, A. V. Virovets, I. V. Kalinina, and D. Yu. Naumov, Izv. Akad. Nauk, Ser. Khim., 2000, 18 [Russ. Chem. Bull., Int. Ed., 2000, 49, 19].
V. P. Fedin, M. N. Sokolov, O. A. Geras'ko, B. A. Kolesov, V. E. Fedorov, A. V. Mironov, D. S. Yufit, Yu. L. Slovohotov, and Yu. T. Struchkov, Inorg. Chim. Acta, 1990, 175, 217.
G. M. Sheldrick, SHELX-97 Release 97-2, University of Göttingen, Germany, 1998.
A. V. Virovets and N. V. Podberezskaya, Kristallografiya, 1992, 37, 1017 [Sov. Phys.-Crystallogr., 1992, 37 (Engl. Transl.)].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fedin, V.P., Kalinina, I.V., Virovets, A.V. et al. Synthesis and structure of the polymeric cluster compound [Mn(H2O)4]3[W4S4(CN)12]·nH2O containing very large cavities. Russian Chemical Bulletin 50, 1525–1528 (2001). https://doi.org/10.1023/A:1013057712434
Issue Date:
DOI: https://doi.org/10.1023/A:1013057712434