Abstract
Purpose. To measure and compare the penetration of cefaclor from the plasma compartment into the interstitial space of lung and skeletal muscle in rats and to integrate the data in a pharmacokinetic model.
Methods. Unbound interstitial concentrations in muscle and lung were measured by in vivo microdialysis following i.v. bolus doses of 50 and 75 mg/kg cefaclor. Unbound muscle concentrations were also measured after a primed, continuous i.v. infusion at an infusion rate of 0.3 mg/kg/min.
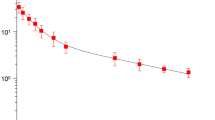
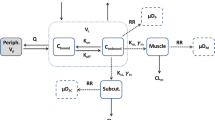
Results. The cefaclor half-life in plasma, muscle and lung was approximately 1 h. Unbound cefaclor concentrations in muscle and lung were found to be virtually identical. A 2-compartment body model was fitted to the data with a tissue penetration factor (AUCtissue(unbound)/AUCplasma(unbound)) of approximately 0.26 independent of dose, tissue and mode of administration.
Conclusions. Unbound concentrations of cefaclor in the interstitial space fluid of lung and skeletal muscle are of similar magnitude and lower than those in plasma. Using total plasma concentrations would overestimate the antibacterial activity of the drug and therefore its clinical efficacy. Instead, therapeutically active levels of cefaclor at the site of action should be taken into account. Microdialysis allows direct measurement of these unbound concentrations.
Similar content being viewed by others
REFERENCES
T. Dalla Costa, A. Nolting, A. Kovar, and H. Derendorf. Determination of free interstitial concentrations of piperacillin-tazobactam combinations by microdialysis. J. Antimicrob. Chemother. 42:769–78 (1998).
Y. DeGuchi, T. Terasaki, H. Yamada, and A. Tsuji. An application of microdialysis to drug tissue distribution study: in vivo evidence for free-ligand hypothesis and tissue binding of beta-lactam antibiotics in interstitial fluids. J. Pharmacobiodyn. 15:79–89 (1992).
A. Nolting, T. Dalla Costa, R. Vistelle, K. H. Rand, and H. Derendorf. Determination of Free Extracellular Concentrations of Piperacillin by Microdialysis. J. Pharm. Sci. 85:369–372 (1996).
A. Kovar, T. Dalla Costa, and H. Derendorf. Comparison of plasma and free tissue levels of ceftriaxone in rats by microdialysis. J. Pharm. Sci. 86:52–56 (1996).
G. R. Fleisher, C. M. Wilmott, J. M. Campos. Amoxicillin combined with clavulanic acid for the treatment of soft tissue infections in children. Antimicrob. Agents. Chemother. 24:679–681 (1983).
C. Joukhadar, M. Frossard, B. X. Mayer, M. N. Brunner, Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385–391 (2001).
J. Martin-Villacorta and R. Mendez, Effect of Temperature and Mobile Phase Composition on RP-HPLC Separation of Cephalosporins. J. Liq. Chrom. 13:3269–3288 (1990).
J. Blanchard. Evaluation of the relative efficacy of various techniques for deproteinizing plasma samples prior to high-performance liquid chromatographic analysis. J. Chromatogr. 226:455–460 (1981).
S. W. Baertschi, D. E. Dorman, J. L. Occolowitz, M. W. Collins, L. A. Spangle, G. A. Stephenson, and L. J. Lorenz. Isolation and Structure Elucidation of the Major Degradation Products of Cefaclor formed under Aqueous Acidic Conditions. J. Pharm. Sci. 86:526–539 (1997).
M. A. Foglesong, J. W. Lamb, and J. V. Dietz, Stability and blood level determinations of cefaclor, a new oral cephalosporin antibiotic. Antimicrob. Agents Chemother. 13:49–52 (1978).
A. P. Gillett, J. M. Andrews, and R. Wise. Comparative in vitro microbiological activity and stability of cefaclor. Postgrad. Med. J. 55:9–11 (1979).
S. W. Baertschi, D. E. Dorman, J. L. Occolowitz, L. A. Spangle, M. W. Collins, M. E. Wildfeuer, and L. J. Lorenz. Isolation and structure elucidation of a novel product of the acidic degradation of cefaclor. J. Pharm. Sci. 82:622–626 (1993).
H. Sourgens, H. Derendorf, and H. Schifferer, Pharmacokinetic profile of cefaclor. Int. J. Clin. Pharm. Ther. 35:374–380 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
de la Penña, A., Dalla Costa, T., Talton, J.D. et al. Penetration of Cefaclor Into the Interstitial Space Fluid of Skeletal Muscle and Lung Tissue in Rats. Pharm Res 18, 1310–1314 (2001). https://doi.org/10.1023/A:1013042128791
Issue Date:
DOI: https://doi.org/10.1023/A:1013042128791