Abstract
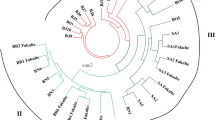
Commercial productivity of watercress (Rorippa nasturtium-aquaticum) can be adversely affected by the pathogenic crook-root fungus, Spongospora subterranea f.sp. nasturti, and watercress viruses. As there are no effective control measures for these diseases, attempts have been made to breed varieties resistant to the crook-root pathogen. This work has been hindered by a lack of knowledge of the genetic base of commercial watercress, and the genetic distance between watercress and allied Brassicaceae which have been identified as candidates for hybridisation programmes. We measured the diversity within these two groups using the RAPD-PCR fingerprinting technique and analysed the data by both distance methods and principal co-ordinate analysis. Little genetic diversity was found within commercial watercress populations. However, watercress formed a unique cluster genetically distinct from other Rorippa species, but equidistant to Cardamine species. It was placed closer to Barbarea verna.
Similar content being viewed by others
References
Al-Shehbaz, I.A. & R.A. Price, 1998. Delimitation of the genus Nasturtium (Brassicacea).Novon 8: 124–126.
Arnold, D.L., A. Flegmann & J.M. Clarkson, 1995. Somaclonal variation inwatercress for resistance to crook root disease. Plant Cell Rep 14: 241–244.
Caetano-Annollés, G., B.J. Bassam & P.M. Gresshoff, 1992. Finger printing genomes with arbitrary oligonucleotide primers. Mol Biol 11: 1–6.
Clapham, A.R., T.G. Tutin & E.F. Warburg, 1962. Flora of the British Isles. Second Edition. CambridgeUniversity Press.
Claxton, J.R., D.L. Arnold, J.M. Clarkson & D. Blakesley, 1998. The regeneration and screeningof watercress somaclones for resistance to Spongospora subterranea f.sp. nasturtii and measurement of somaclonal variation. Plant Cell Tiss Org Cult 52: 155–164.
Crouch, J.H., B.G. Lewis, D.J. Lydiate & R. Mithen, 1995.Genetic diversity of wild, weedy and cultivated forms of Brassica rapa. Heredity 74: 491–496.
Dellaporta, S.L.,J. Wood & J.B. Hicks, 1983. A plant DNA minipreparation; version II. Plant Mol Biol Rep 1: 19–21.
Felsenstein, J., 1989. PHYLIP - Phylogeny inference package (version 3.2). Cladistics 5: 164–166.
Gower, J.C., 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53: 325–338.
Gower, J.C., 1971. A general coefficient of similarity and some of its properties.Biometrics 27: 857–874.
Howard, H.W. & I. Manton, 1946. Autopolyploid and allopolyploid watercresswith the description of a new species. Ann Bot 10: 1–13.
Jaccard, P., 1901. Etude comparative la distributionflorale dans une portion des Alpes et des Jura. Bull Soc Vaudoise Sci Natl 37: 547–579.
Jain, A., S. Bhatia, S.S. Banga, S. Prakash & M. Lakshikuraran, 1994. Potential use of random amplified polymorphic DNA (RAPD) technique to study the genetic diversity in Indian mustard (Brassica juncea) and its relationship to heterosis. Theor Appl Genet 88: 116–122.
Les, D.H., 1994. Molecular systematics and taxonomy of the lake cress (Neobeckia aquatica;Brassicaceae), an imperiled aquatic mustard. Aquat Bot 49: 149–165.
Mailer, R.J., R. Scarth & B. Fristensky,1994. Discrimination among cultivars of rapeseed (Brassica napus L.) using DNA polymorphisms amplified from arbitary primers. Theoret Appl Genet 87: 697–704.
Marmey, P., J.R. Beeching, S. Hamon & A. Charrier, 1994.Evaluation of cassava (Manihot esculenta Crantz) germplasm collections using RAPD markers. Euphytica 74: 203–209.
Nei, M., 1987. Molecular Evolutionary Genetics. Columbia University Press, New York, Guildford, Surrey.
Rich, T.C., 1991. Crucifers of Great Britain and Ireland. BSBS handbook No 6. The Cambrian Printers, Aberystwyth.
Saitou, J. & M. Nei, 1987. The neighbor-joining method: A new method for reconstructingphylogenetic trees. Mol Biol Evol 4: 406–425.
Sambrook, J., E.F. Fritsch & T. Manniatis, 1989.Molecular Cloning; a laboratory manual. Second edition Cold Spring harbor laboratory Press, Cold Spring Harbor, NY.
Sneath, P.H.A. & R.R. Sokal, 1973. In: Numerical Taxonomy, pp 230–234. WH Freeman Company, SanFransisco, CA, USA.
Sokal, R. & C.D. Michener, 1958. A statistical method for evaluating systematicrelationships. Uni Kans Sci Bull 38: 1409–1438.
Spencer, D.M. & H.H. Glascock, 1953. Crook-rootdisease of watercress. Plant Pathol 2: 19.
Tomlinson, J.A., 1958. Crook root of watercress III; The causalorganism Spongospora subterranea (Wallr.) Lagerh f. sp. nasturtii f. sp. Nov. Trans Br Mycol Soc 41(4): 491–498.
Walsh, J.A., 1992. Resistant watercress. Grower, August 6th: 18–21
Walsh, J.A., C.M. Clay & A. Miller, 1989a. A new virus disease of watercress in England. Bull OEPP 19: 463–470.
Walsh, J.A.,C.M. Clay, A. Miller & J.C. Rowe, 1989b. A fungustransmitted virus of watercress. Aspects Appl Biol 22: 101–108.
Williams, J.G.K., A.R. Kubelik, K.J. Livak, J.A. Rafalski & S.V. Tingey, 1990. DNApolymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18: 6531–6535.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sheridan, G., Claxton, J., Clarkson, J. et al. Genetic diversity within commercial populations of watercress (Rorippa nasturtium-aquaticum), and between allied Brassicaceae inferred from RAPD-PCR. Euphytica 122, 319–325 (2001). https://doi.org/10.1023/A:1012913820046
Issue Date:
DOI: https://doi.org/10.1023/A:1012913820046