Abstract
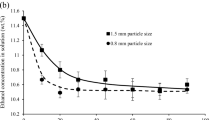
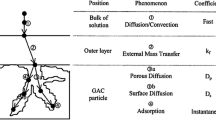
The sorption of acid dyes from aqueous effluents onto activated carbon has been studied. The effects of initial dye concentration and activated carbon mass on the rate of Acid Blue 80, Acid Red 114 and Acid Yellow 117 removal have been investigated. A three-resistance mass transport model based on film, pore and surface diffusion control has been applied to model the concentration decay curves. The model incorporates an effective diffusion coefficient D eff, which is dependant on the equilibrium solid phase concentration or fractional surface coverage. The results of the film-pore-surface diffusion model are compared with the data obtained from the basic film-pore diffusion model. It has been found that the film-pore-surface diffusion model provides a major improvement over the data correlated by the film-pore diffusion model. Also, the relationship between surface diffusion and fractional surface coverage has been investigated for the adsorption of acid dyes on activated carbon.
Similar content being viewed by others
References
Al-Asheh, S., F. Banat, R., Al-Omari, and Z. Duvnjak, “Predictions of Binary Sorption Isotherms for the Sorption of Heavy Metals by Pine Bark Using Single Isotherm Data,” Chemosphere, 41, 659–665 (2000).
Al Duri, B., G. McKay, and M.S. El Geundi, “Three-Resistance Transport Model for Dye Adsorption Onto Bagasse Pith,” J. Envir. Eng., 116, 487–502 (1990).
Asfour, H.M., M.M. Nassar, O.A. Fadali, and M.S. El-Geundi, “Colour Removal from Textile Effluents Using Hardwood Sawdust as an Adsorbent,” J. Chem. Technol. Biotechnol., 35, 28–35 (1985).
Cheung, C.W., K.H. Choy, J.F. Porter, and G. McKay, “Combined Diffusion Model for Batch Adsorption,” Ads. Sci.Technol., 37, 426–430 (2000).
Crittenden, J.C., P. Luft, D.W. Hand, J.L. Oravltz, S.W. Loper, and M. Arl, “Solid Pore Diffusion Model for Adsorption Systems,” Env. Sci. azTechnol., 19, 1037–1043 (1985).
Darken, L.S., “Diffusion, Mobility and Their Interrelation Through Free Energy in Binary Metallic Systems,” Trans. AIME, 175, 184–201 (1948).
Do, H.D., I. Prasetyo, and D.D. Do, “Surface Diffusion of Hydrocabon in Activated Carbon,” Ads. Sci. Technol., 37, 184–188 (2000).
Do, D.D. and R.G. Rice, “On the Relative Importance of Pore and Surface Diffusion in Non-Equilibrium Adsorption Rate Processs,” Chem. Eng. Sci., 42, 2269–2284 (1987).
El-Geundi, M.S., “Mass Transfer Processes During Colour Removal from Effluents Using Adsorption Techniques,” Ph.D. Thesis, Minia University, Egypt, 1987.
El-Geundi, M.S. and M. Akl, “Colour Removal From Textile Effluents by Adsorption Techniques,” Water Research, 25, 271–273 (1991).
Fritz, W. and E.U. Schlünder, “Competitive Adsorption of Two Dissolved Organics Onto Activated Carbon-I,” Chem. Eng. Sci., 36, 721–730 (1981).
Furusawa, T. and J.M. Smith, “Fluid-Particle and Intraparticle Mass Transport Rates in Slurries,” Ind. Eng. Chem. Fundam., 12, 197–203 (1973).
Hand, D.W., J.C. Crittenden, and W.E. Thacker, “User Orientated Batch Reactor Solutions to the Homogeneous Surface Diffusion Model.” J. Envir., Engngl. Div., Am. Soc. Civ. Engrs., 109, 82–92 (1983).
Higashi, K., H. Ito and J. Oishi, “Surface Diffusion Phenomina in Gasous Diffusion, (I). Surface Diffusion of Pure Gas,” J. Atom. Energy Soc. Japan, 5, 846–853 (1963).
Hu, X. and D.D. Do, “Effect of Surface Heterogeneity on the Adsorption Kinetics of Gases in Activated Carbon: Pore Size Distribution vs Energy Distribution,” Langmuir, 10(9), 3296–3302 (1994).
Kapoor, A. and R.T. Yang, “Surface Diffusion on Energetically Heterogeneous Surfaces-An Effective Medium Approximation Approach,” Chem. Eng. Sci., 31, 129–135 (1990).
Komiyama, H. and J.M. Smith, “Surface Diffusion in Liquid Filled Pores,” AIChE J., 20, 1110–1177 (1974).
Langmuir, I., “The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum,” J. Amer. Chem. Soc., 40, 1361–1403 (1918).
Maghami, G.G. and G.A. Roberts, “Studies on the Adsorption of Anionic Dyes on Chitosan,” Makromol. Chem., 189, 2239–2243 (1988).
Marshall, W.E. and E.T. Champagne, “Agricultural By-products as Adsorbents for Metal Ions in Laboratory Prepared Solutions and in Manufacturing Wastewater,” J. Env. Sci. Health Part A, A30, 241–261 (1995).
Marshall, W.E., E.T. Champagne, and W.J. Evans, “Use of Rice Milling By-products (Hulls and Bran) to Remove Metal Ions From Aqueous Solutions,” J. Env. Sci. Health Part A, A28, 1977–1992 (1993).
Marshall, W.E. and M.M. Johns, “Agricultural By-products as Metal Adsorbents: Sorption Properties and Resistance to Mechanical Abrasion,” J. Chem. Technol. Biotechnol., 66, 192–198 (1996).
McKay, G. and S.J. Allen, “Surface Mass Transfer Processes Using Peat as an Adsorbent for Dyestuffs,” Canadian J. Chem. Eng., 58, 521–526 (1980).
McKay, G. and S.J. Allen, “Pore Diffusion Model for Dye Adsorption Onto Peat in Batch Adsorbers,” Canadian J. Chem. Eng., 62, 340–345 (1984).
McKay, G., S.J. Allen, I.F. McConvey, and M.S. Otterburn, “Transport Processes in the Sorption of Colored Ions by Peat Particles,” J. Coll. Interf. Sci., 80, 323–339 (1981).
McKay, G., S.J. Allen, and L. Whitten, “The Production and Characterisation of Activated Carbons: A Review,” Dev, Chem. Eng. Mineral Process, 6(5), 231–261 (1998).
McKay, G., H.S. Blair, and J.R. Gardner, “Adsorption of Dyes on Chitin. I. Equilibrium Studies,” J. Appl. Poly. Sci., 27, 3043–3057 (1982).
McKay, G., H.S. Blair, and J.R. Gardner, “The Adsorption of Dyes in Chitin. III. Intraparticle Diffusion Processes,” J. Appl. Poly. Sci., 28, 1767–1778 (1983).
McKay, G., M. El Geundi, and M.M. Nassar, “Equilibrium Studies During the Removal of Dyestuffs From Aqueous Solutions Using Pith,” Water Research, 21, 1513–1520 (1987).
McKay, G., M. El Geundi, and M.M. Nassar, “Pore Diffusion During the Adsorption of Dyes Onto Bagasse Pith,” Trans. IChemE., 74, 277–288 (1996).
McKay, G., M.S. Otterburn, and J.A. Aga, “Fuller's Earth and Fired Clay as Adsorbents for Dyestuffs Equilibrium and Rate Studies,” Water, Air, Soil Pollut., 24, 307–322 (1985).
Namasivayam, C. and D.J.S.E. Arasi, “Removal of Congo Red From Wastewater by Adsorption OntoWaste Red Mud,” Chemosphere, 34, 401–417 (1996).
Namasivayam, C. and N. Kanchana, “Waste Banana Pith as an Adsorbent for Colour Removal From Wastewaters,” Chemosphere, 25, 1691–1705 (1992).
Namasivayam, C., N. Muniasamy, K. Gayatri, M. Rani, and K. Ranganathan, “Removal of Dyes From Aqueous Solutions by Cellulosic Waste Orange Peel,” Bioresource Technol., 57, 37–43 (1996).
Nassar, M.M., M.F. Hamoda, and G.H. Radwan, “Adsorption Equilbiria of Basic Dyestuff Onto Palm-Fruit Branch Particles,” Wat. Sci. Technol., 32, 27–32 (1995).
Neogi, P., and E. Ruckenstein, “Transport Phenoma in Solids with Bidispersed Pores,” AIChE J., 26, 787–799 (1980).
Neretnieks, I., “Adsorption of Components Having a Saturation Isotherm,” Chem. Ing. Technol., 46, 781–792 (1974).
Okieimen, F.E., E.U. Okundia, and D.E. Ogbeifun, “Sorption of Cadmium and Lead Ions on Modified Groundnut (Arachis Hypogea) Husks,” J. Chem. Technol. Biotechnol., 51, 97–103 (1991).
Peel, R.G., A. Benedek, and C.M. Crowe, “A Branched Pore Kinetic Model for Activated Carbon Adsorption,” AIChE J., 27(1), 26–31 (1981).
Perineau, F., J. Molinier, and A. Gaset, “Adsorption of Ionic Dyes on Wool Carbonizing Waste,” Water Research, 17, 559–567 (1983).
Seo, T., T. Kanbara, and T. Iijima, “Sorption of Methyl Orange by Chitosan Gels Having Hydrophobic Groups,” J. Appl. Polym. Sci., 36, 1443–1451 (1988).
Spahn, H. and E.U. Schlünder, “The Scale-Up of Activated Carbon Columns for Water Purification, Based on Results From Batch Test-I,” Chem. Eng. Sci., 30, 529–537 (1975).
Suzuki, M. and T. Fujii, “Concentration Dependence of Surface Diffusion Coefficient of Propionic Acid in Activated Carbon,” AIChE J., 28, 380–388 (1982).
Traegner, U.K. and M.T. Suidan, “Evaluation of Surface and Film Diffusion Coefficients for Carbon Adsorption,” Wat. Res., 23(3), 267–273 (1988).
Whitaker, S., “Diffusion in Packed Beds of Porous Particles,” AIChE J., 34, 679–688 (1988).
Yang, R.T., J.B. Fenn, and G.L. Haller, “Modification to the Higashi Model for Surface Diffusion,” AIChE. J., 19, 1052–1054 (1973).
Yeroshenkova, G.V., S.A. Volkov, and K.I. Sakodynskii, “Effect of Packing Irregularities Along the Bed Length,” J. Chromatog., 262, 19–24 (1983).
Yoshida, H., N. Kishimoto, and T. Kataoka, “Adsorption of Strong Acid on Polyaminated Highly Porous Chitosan: Equilibria,” Ind. Eng. Chem. Res., 33, 854–859 (1994).
Yoshida, H., N. Kishimoto, and T. Kataoka, “Adsorption of Glutamic Acid on Polyaminated Highly Porous Chitosan; Equilibria,” Ind. Eng. Chem. Res., 34, 347–355 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Choy, K.K., Porter, J.F. & Mckay, G. A Film-Pore-Surface Diffusion Model for the Adsorption of Acid Dyes on Activated Carbon. Adsorption 7, 231–247 (2001). https://doi.org/10.1023/A:1012736918283
Issue Date:
DOI: https://doi.org/10.1023/A:1012736918283