Abstract
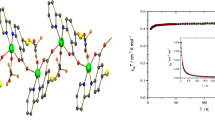
A new copper carboxylate polymer with cyanoacetate anion as a ligand was synthesized and studied using X-ray diffraction, IR, and EPR spectroscopy. The crystal is tetragonal: a= 14.702(2) Å, c= 13.470(3) Å, Z= 8, space group I41/a, and R= 0.0634. The copper atoms in the centrosymmetric dimeric fragment have a square-pyramidal surrounding with the CuO4N coordination core and are joined through four bidentate bridging anions of cyanoacetic acid Cu(1)"–O(1A) 1.931(4) Å, Cu(1)"–O(1B) 1.926(4) Å, Cu(1)–O(2B) 2.018(3) Å, Cu(1)–O(2A) 2.036(4) Å, and Cu(1)–N(1A)" 2.206(5) Å). The Cu···Cu" distance in the dimer is 2.709 Å. The copper atom is extended from the mean equatorial plane toward the axial nitrogen atom by 0.23 Å. EPR data confirm strong antiferromagnetic interaction (2J≈ –275 cm–1) between the copper(II) ions of the dimeric fragment, whereas the interaction between the dimers is significantly weaker (J< 0.3 cm–1).
Similar content being viewed by others
REFERENCES
Yablokov, Yu.V., Voronkova, V.K., and Mosina, L.V., Paramagnitnyi rezonans obmennykh klasterov (Paramagnetic Resonance of Exchange Clusters), Moscow: Nauka, 1988.
Simonov, Yu.A., Yablokov, Yu.V., and Milkova, L.N., Kristallicheskie struktury neorganicheskikh soedinenii (Crystal Structures of Inorganic Compounds), Malinovskii, T.I., Ed., Kishinev: Shtiintsa, 1974, p. 61.
Porai-Koshits, M.A., Itogi Nauki Tekh., Ser.: Kristallokhim., 1981, vol. 15, p. 3.
Mehrota, R.C. and Bohra, R., Metal Carboxilates, London: Academic, 1983.
Catterick, J. and Thornton, P., Adv. Inorg. Chem. Radiochem., 1977, vol. 20, p. 291.
Oldhom, C., Prog. Inorg. Chem., 1968, vol. 10, p. 223.
Doedens, R.J., Prog. Inorg. Chem., 1976, vol. 21, p. 209.
Melnik, M., Coord. Chem. Rev., 1981, vol. 36, no. 1, p. 1.
Bleaney, B. and Bowers, R.D., Proc. R. Soc. London, Ser. A, 1952, vol. 214, p. 451.
Niekerk, J.N., and van Schoening, F.R.L., Acta Crystallogr., 1953, vol. 6, no. 3, p. 227.
Meenakumori, S., Lakshminarayngu, M., Tiwarg, S.K., and Chakravaty, A.K., Inorg. Chem., 1995, vol. 34, no. 20, p. 5091.
Gradziki, A., Szluk, E., and Wojtezok, A., Polyhedron, 1999, vol. 18, nos. 3-4, p. 519.
Dendrinju-Samara, C., Promas, G., and Christophorou, K., J. Chem. Soc., Dalton Trans., 1996, no. 18, p. 3737.
Ablov, A.V., Simonov, Yu.A., and Malinovskii, T.I., Dokl. Akad. Nauk SSSR, 1966, vol. 171, no. 4, p. 854.
Simonov, Yu.A. and Malinovskii, T.I., Kristallografiya, 1970, vol. 15, no. 2, p. 370.
Bird, M.J. and Lomer, T.R., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, vol. 28, no. 2, p. 242.
Yawney, D.B.W. and Doedens, R.J., J. Am. Chem. Soc., 1970, vol. 92, no. 21, p. 6350.
O'Connor, B.N. and Maslen, E.N., Acta Crystallogr., 1966, vol. 20, no. 6, p. 824.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 1990, vol. 46, no 6, p. 467.
Sheldrick, G.M., SHELXL93: Program for the Refinement of Crystal Structure, Göttingen: Univ. of Göttingen, 1993.
Neiland, O.Ya., Organicheskaya khimiya (Organic Chemistry), Moscow: Vysshaya Shkola, 1990.
Nakomoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, New York: Wiley, 1986. Translated under the title IK-spektry i spektry KR neorganicheskikh i koordinatsionnykh soedinenii, Moscow: Mir, 1991.
Dolphin, D. and Wick, A., Tabulation of Infrared Spectral Data, New York: Wiley, 1977.
Bencini, A. and Gatteschi, D., Electron Paramagnetic Resonance of Exchange Coupled Systems, Berlin: Springer-Verlag, 1990.
Abragam, A. and Bleaney, B., Electron Paramagnetic Resonance of Transition Ions, Oxford: Clarendon, 1970, vol. 1.
Voronkova, V.K., Mosina, L.V., and Yablokov, Yu.V., Mol. Phys., 1992, vol. 75, p. 1275.
Smith, T.D. and Pilbrow, J.R., Coord. Chem. Rev., 1974, vol. 13, p. 17.
Van Vleck, J.H., The Theory of Electric and Magnetic Susceptibilities, Oxford: Oxford Univ. Press, 1932, p. 182.
Figgis, B.N. and Martin, R.L., J. Chem. Soc., 1956, no. 10, p. 3837.
Anderson, P.W., J. Phys. Soc. Jpn., 1954, vol. 9, no. 2, p. 316.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Novitskii, G., Shova, S., Voronkova, V.K. et al. Copper(II) Cyanoacetate Polymer: Synthesis and Structure. Russian Journal of Coordination Chemistry 27, 791–795 (2001). https://doi.org/10.1023/A:1012567022543
Issue Date:
DOI: https://doi.org/10.1023/A:1012567022543