Abstract
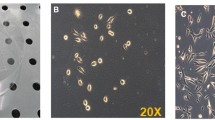
The method for obtaining human myoblast culture has been modified to consider the specific histological localization of the satellite cells as well as their growth properties; the cultivation conditions have been selected to grow up to 150000 cells/cm2. At high densities, the cells remain mononuclear and preserve their typical myoblast morphology as well as the capacity for fusion and the formation of myotubes. By contrast to fibroblasts, up to 80% of the cells in the myoblast culture were positive in the acid phosphatase test, which indicates their stem nature. The obtained myoblast cultures were used in the clinical tests of cell-mediated gene therapy of Duchenne's muscular dystrophy as well as in the bioassay for the effects of biologically active compounds.
Similar content being viewed by others
REFERENCES
Allbrook, D., Skeletal Muscle Regeneration, Muscle Nerve, 1981, vol. 4, pp. 234-245.
Anderson, J.E., A Role for Nitric Oxide in Muscle Repair: Oxide-Mediated Activation of Muscle Satellite Cells, Mol. Biol. Cell, 2000, vol. 11, pp. 1859-1874.
Beggs, A.H.,Koenig, M.,Boyce, F.M., andKunkel, L.M., Detection of 98% of DMD/BMD Gene Deletions by Polymerase Chain Reaction, Hum. Genet., 1990, vol. 86, pp. 45-48.
Chamberlain, J.S.,Gibbs, R.A.,Ranier, J.E., et al., Deletion Screening of the Duchenne Muscular Dystrophy Locus via Multiplex DNA Amplification, Nucl. Acids Res., 1988, vol. 16, pp. 11141-11156.
Chernikov, V.T.,Shishkin, S.S.,Krokhina, T.B., et al., Express Bioassay for Cytotoxic Effects of Biologically Active Compounds (BAC) Based on Human Fibroblast Culture, Byul. Eksperim. Biol. Med., 2000, no. 5, pp. 587-590.
Diagnostic Criteria for Neuromuscular Disorders, Emery,?A.E.H., Ed., Baarn: European Neuromuscular Centre, 1994.
Garrels, J.I., Changes in Protein Synthesis during Myogenesis in Clonal Cell Line, Dev. Biol., 1979, vol. 73, pp. 134-152.
Hohlfeld, R. andEngl, A.G., The Immunobiology of Muscle, Immunol. Today, 1994, vol. 15. P. 269-274.
Knox, P.,Uphill, P.F.,Fry, J.R., et al. The FRAME Multicentre Project in vitro Cytotoxicology, Fd. Chem. Toxic., 1986, vol. 24, no. 6/7, pp. 457-463.
Knuh, J., Effects of Sodium Butyrate, a New Pharmacological Agent, on Cells in Culture, Mol. Cell. Biochem., 1982, vol. 42, pp. 65-82.
Kovaleva, M.A., Kovalev, L.I., Khudaidatov, A.I., et al., Comparative Analysis of Protein Pattern in Human Skeletal and Heart Muscle by Two-dimensional Electrophoresis, Biokhimiya, 1994, vol. 59, pp. 675-681.
Kovalyov, L.I.,Shishkin, S.S.,Efimochkin, A.S., et al., The Mayor Protein Expression Profile and Two-dimensional Protein Database of Human Heart, Electrophoresis, 1995. V. 16. P. 1160-1169.
Krakhmaleva, I.N.,Lipatova, N.A.,Shishkin, S.S., andShakhovskaya, N.I., Study of Changes in Dystrophin Gene Structure in Duchenne Muscular Dystrophy Patients, Zhurn. Nevrol. Psikhiatr., 1999, vol. 99, no. 3, pp. 41-43.
Krochina, T.B.,Raevskaja, G.B., andShishkin, S.S., Tissue Therapy for Treatment of Primary Myodystrophies, New Developments and New Applications in Animal Cell Technology, Merten, O.W.,Perrin, P., andGriffiths, B., Eds., Kluwer Academic, 1998, pp. 677-679.
Krokhina, T.E.,Shishkin, S.S.,Raevskaya, G.B., et al., Specific Pattern of Gene Expression in Human Myoblasts Revealed by Analysis of the Primary and Cloned Cell Cultures, Byul. Eksperim. Biol. Med., 1996, vol. 122, no. 9, pp. 314-317.
Law, P.K., Myoblast Transfer: Gene Therapy for Muscular Dystrophy, CRC, 1994.
Law, P.K.,Goodwin, T.G.,Fang, Q., et al. First Human Myoblast Transfer Therapy Continues to Show Dystrophin after 6 Years, Cell Transplant., 1997, vol. 6, no. 1, pp. 95-100.
Lindblom, B. andHolmlung, G., Rapid Purification for Restriction Fragment Length Polymorphism Analysis, Gene Anal.Techn., 1988, vol. 5, pp. 97-101.
Linge, C.,Green, M.R., andBrooks, R.F., A Method for Removal Fibriblasts from Human Tissue Culture Systems, Exp. Cell Res., 1989, vol. 185, pp. 519-528.
Mendell, J.R.,Kissel, J.T.,Amato, A.A., et al., Myoblast Transfer in the Treatment of Duchenne's Muscular Dystrophy, New Eng. J. Med., 1995, vol. 333, pp. 832-838.
Morgan, J.E., Myogenicity in vitro and in vivo Mouse Muscle Cells Separated on Discontinuous Percll Gradients, J.?Neurol. Sci., 1988, vol. 85, pp. 197-207.
Rando, T.A. andBlau, H.M., Primary Mouse Myoblast Puri-fication, Characterization and Transplantation for Cell-Mediated Gene-Therapy, J. Cell. Biol., 1994, vol. 125, pp. 1275-1287.
Repin, V.S. andSukhikh, T.G., Meditsinskaya kletochnaya biologiya (Medical Cell Biology), Moscow: BEBiM, 1998.
Shishkin, S.S. Nasledstvennye nervno-myshechnye bolezni (Genetic Neuromuscular Diseases), Moscow: VINITI, 1997.
Shishkin, S.S.,Krokhina, T.B.,Terekhov, S.M., et al., First Instance of Normal Donor Myoblasts Transplantation to a Duchenne Muscular Dystrophy Patient in Russia (Cell Therapy by the “Single Muscle Treatment” Scheme), Byul. Ross. Obshch. Med. Genetikov, 1999, no. 2, pp. 15-16.
Shishkin, S.S.,Shakhovskaya, N.I.,Lunga, I.N., et al., Genetic Neuromuscular Diseases, Certa in Problems of Treatment and Help to Affected Families, Mnogolikost’ sovremennoi genetiki cheloveka (Versatile Modern Human Genetics), Shishkina, S.S. Ed., Moskva: Gilem, 2000, pp. 197-276.
Shishkin, S.S.,Terekhov, S.M.,Krokhina, T.D., et al., First Clinical Testing of Cell-Mediated Gene-Therapy of Duchenne Muscular Dystrophy in Russia, Tez. dokl. VIII Vseros. s'ezd nevrologov, 2001.
Skehan, P.,Storeng, R.,Ascudiero, D., et al., New Colorimetric Cytotoxicity Assay for Antycancer-Drug Screening, J. Natl. Cancer Inst., 1990, vol. 82, pp. 1107-1112.
Svensson, E.C.,Tripathy, S.K., andLeiden, J.M., Muscle-Based Gene Therapy: Realistic Possibilities for the Future, Mol. Med. Today, 1996, April, pp. 166-172.
Testing Outward Application Drugs in Human Epidermal Cell Culture, in Metodicheskie rekomendatsii no. 96/247 (Methodical Recommendations No. 96/247), Moscow: Minzdrav, 1996.
Walsh, F.S. andRitter, M.A., Surface Antigen Differentiation during Human Myogenesis in Culture, Nature, 1984, vol. 289, pp. 60-64.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Terekhov, S.M., Krokhina, T.B., Shishkin, S.S. et al. Human Myoblast Culture as Muscle Stem Cells in Medical and Biological Studies. Biology Bulletin 28, 630–635 (2001). https://doi.org/10.1023/A:1012380505528
Issue Date:
DOI: https://doi.org/10.1023/A:1012380505528