Abstract
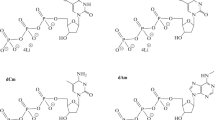
The interaction of dNTPs with the active site of HIV-1 reverse transcriptase (HIV RT) has been investigated. The kinetic parameters of primer elongation catalyzed by wild-type HIV-1 RT and two of its mutants with substitutions for Tyr115 using dTTP and two of its photoreactive analogs were determined. The substitution for Tyr115 with alanine or tryptophan resulted in an increase in K m values of dTTP and its analogs. Wild-type RT and its mutants were photoaffinity modified using photoreactive primer synthesized in situ. The modification was made in two variants: direct photocross-linking under UV irradiation and photosensitized modification using Pyr-dUTP as a sensitizer. The use of the sensitizer decreased the number of modification products and increased selective labeling of the catalytic subunit of both the mutant and wild-type RT.
Similar content being viewed by others
REFERENCES
Rodgers, D. W., Gamblin, S. J., Harris, B. A., Ray, S., Culp, J. S., Hellmig, B., Woolf, D. J., Debouck, C., and Harrison, S. C. (1995) Proc.Natl.Acad.Sci.USA, 92, 1222–1226.
Jacobo-Molina, A., Ding, J., Nanni, R. G., Clark, A. D., Lu, Jr. X., Tantillo, C., Williams, R. L., Kamer, G., Ferris, A. L., Clark, P., Hizi, A., Hughes, S. H., and Arnold, E. (1993) Proc.Natl.Acad.Sci.USA, 90, 6320–6324.
Kohlstaedt, L. A., Wang, J., Friedman, J. M., Rice, P. A., and Steitz, T. A. (1992) Science, 256, 1783–1790.
Huang, H., Chopra, R., Verdine, G. L., and Harrison, S. C. (1998) Science, 282, 1669–1674.
Martín-Hernández, A. M., Domingo, E., and Menéndez-Arias, L. (1996) EMBO J., 15, 4434–4442.
Martín-Hernández, A. M., Gutiérrez-Rivas, M., Domingo, E., and Menéndez-Arias, L. (1997) Nucleic Acids Res., 25, 1383–1389.
Cases-González, C. E., Gutiérrez-Rivas, M., and Menéndez-Arias, L. (2000) J.Biol.Chem., 275, 19759–19767.
Harris, D., Kaushik, N., Pandey, P. K., Yadav, P. N., and Pandey, V. N. (1998) J.Biol.Chem., 273, 33624–33634.
Gutiérrez-Rivas, M., Ibánez, A., Martínez, M. A., Domingo, E., and Menéndez-Arias, L. (1999) J.Mol.Biol., 290, 615–625.
Cheng, N., Merrill, B. M., Painter, G. R., Frick, L. W., and Furman, P. A. (1993) Biochemistry, 32, 7630–7634.
Doronin, S. V., Dobrikov, M. I., Buckle, M., Roux, P., Buc, H., and Lavrik, O. I. (1994) FEBS Lett., 354, 200–202.
Shcherbik, N. V., Khodyreva, S. N., Vlasov, V. A., Dobrikov, M. I., Dymshits, G. M., and Lavrik, O. I. (1997) Mol.Biol.(Moscow), 31, 290–297.
Lin, S., Henzel, W. J., Nayak, S., and Dennis, D. (1998) J.Biol.Chem., 273, 997–1002.
Sheng, N., and Dennis, D. (1993) Biochemistry, 32, 4938–4942.
Dobrikov, M. I., Doronin, S. V., Safronov, I. V., Shishkin, G. V., and Lavrik, O. I. (1994) Khimiya v Interesakh Ustoichivogo Razvitiya, 2, 529–534.
Hansen, J., Schulze, T., and Moelling, K. (1987) J.Biol.Chem., 262, 12393–12396.
Bavand, M. R., Wagner, R., and Richmond, T. J. (1993) Biochemistry, 32, 10543–10552.
Lavrik, O. I., Prasad, R., Beard, W. A., Safronov, I. V., Dobrikov, M. I., Srivastava, D. K., Shishkin, G. V., Wood, T. G., and Wilson, S. H. (1996) J.Biol.Chem., 271, 21891–21897.
Kolpashchikov, D. M., Rechkunova, N. I., Dobrikov, M. I., Khodyreva, S. N., Lebedeva, N. A., and Lavrik, O. I. (1999) FEBS Lett., 448, 141–144.
Rechkunova, N. I., Kolpashchikov, D. M., Lebedeva, N. A., Petruseva, I. O., Degtyarev, S. Kh., and Lavrik, O. I. (2000) Biochemistry (Moscow), 65, 244–249.
Bichenkova, E. V., Marks, D. S., Lokhov, S. G., Dobrikov, M. I., Vlassov, V. A., and Douglas, K. T. (1997) J.Biomol.Struct.Dyn., 15, 307–319.
Kolpashchikov, D. M., Aleksandrova, L. A., Zakirova, N. F., Khodyreva, S. N., and Lavrik, O. I. (2000) Bioorg.Khim., 26, 134–137.
Godovikova, T. S., Kolpashchikov, D. M., Orlova, T. N., and Richter, V. A. (1997) FASEB J., 11, 1367.
Kolpashchikov, D. M., Zakharenko, A. L., Dezhurov, S. V., Rechkunova, N. I., Khodyreva, S. N., Degtyarev, S. Kh., Litvak, V. V., and Lavrik, O. I. (1999) Bioorg.Khim., 25, 110–117.
Laemmli, U. K. (1970) Nature, 227, 680–685.
Bradford, M. M. (1976) Analyt.Biochem., 72, 248–254.
Scopes, R. K. (1982) in Protein Purification, Springer-Verlag N.Y. Inc.
Reardon, J. E., and Miller, W. H. (1990) J.Biol.Chem., 265, 20302–20307.
Maniatis, T., Fritsch, E. F., and Sambrook, J. (1982) in Molecular Cloning, Cold Spring Harbor, Laboratory Press, N. Y., p. 125.
Kolpashchikov, D. M., Ivanova, T. M., Bogachev, V. S., Nasheuer, H.-P., Weisshart, K., Favre, A., Pestryakov, P. E., and Lavrik, O. I. (2000) Bioconjugate Chem., 11, 445–451.
El-Deiry, W. S., Donwey, K. M., and So, A. G. (1984) Proc.Natl.Acad.Sci.USA, 81, 7378–7382.
El-Deiry, W. S., So, A. G., and Donwey, K. M. (1988) Biochemistry, 24, 5810–5817.
Beckman, R. A., Mildvan, A. S., and Loeb, L. A. (1985) Biochemistry, 27, 546–553.
Eger, B. T., Kuchta, R. D., Carroll, S. S., Benkovic, P. A., Dahlberg, M. E., Joyce, C. M., and Benkovic, S. J. (1991) Biochemistry, 30, 1441–1448.
Ricchetti, M., and Buc, H. (1993) EMBO J., 12, 387–396.
Goodman, M. F., Keener, S., Guidotti, S., and Branscomb, E. W. (1983) J.Biol.Chem., 258, 3496–3475.
Seal, G., Sheaman, C. W., and Loeb, L. A. (1979) J.Biol.Chem., 254, 5229–5237.
Copeland, W. C., Lam, N. K, and Wang, T. S.-F. (1993) J.Biol.Chem., 268, 11041–11049.
Sirover, M. A., and Loeb, L. A. (1977) J.Biol.Chem., 252, 3605–3610.
Pelletier, H., Sawaya, M. R., Wolfle, W., Wilson, S. H., and Kraut, J. (1996) Biochemistry, 35, 12762–12777.
Tabor, S., and Richardson, C. C. (1989) Proc.Natl.Acad.Sci.USA, 86, 4076–4080.
Brandis, J. W., Edwards, S. G., and Johnson, K. A. (1996) Biochemistry, 35, 2189–2200.
Rechkunova, N. I., Okhapkina, S. S., Anarbaev, R. O., Lokhova, I. A., Degtyarev, S. Kh., and Lavrik, O. I. (2000) Biochemistry (Moscow), 65, 609–614.
Yakubov, L., Khaled, Z., Zhang, L. M., Truneh, A., Vlassov, V., and Stein, C. A. (1993) J.Biol.Chem., 268, 18818–18823.
Smerdon, S. J., Jager, J., Wang, J., Kohlstaedt, L. A., Chirino, A. J., Friedman, J. M., Rice, P. A., and Steitz, T. A. (1994) Proc.Natl.Acad.Sci.USA, 91, 3911–3915.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zakharenko, A.L., Kolpashchikov, D.M., Khodyreva, S.N. et al. Investigation of the dNTP-Binding Site of HIV-1 Reverse Transcriptase Using Photoreactive Analogs of dNTP. Biochemistry (Moscow) 66, 999–1007 (2001). https://doi.org/10.1023/A:1012373626717
Issue Date:
DOI: https://doi.org/10.1023/A:1012373626717