Abstract
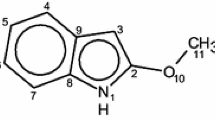
Tautomeric transformations of 4-methyldihydrofuro[2,3-h]coumarin-9-one and its 8-substituted derivatives were studied by 1H NMR, electronic absorption spectroscopy, and quantum chemistry. The 1H NMR spectra of these compounds in CDCl3 show that they exist in the ketone form, and in more polar sol- vents they can pass into the enol form. By electronic absorption spectroscopy it was established that the derivatives containing electron-acceptor substituents in the 8 position of the furanone ring undergo tautomeric transformations as the composition of the solvent is varied from 100% methanol to 100% CCl4. At the same time, the derivatives with electron-donor substituents in the same position do not show any specific alterations in the absorption spectra with solvent. Analogous pattern was observed in the enolization of substituted di- hydrofurocoumarinones by acetylation: In presence of electron-donor substituents in the 8 position, no acetyla- tion occurred, while with the compounds containing electron-acceptor substituents, the corresponding 9-acet- oxy-4-methylangelicins were prepared in high yields. Calculations by the PPP/CI method of the electronic absorption spectra 4-methyldihydrofuro[2,3-h]coumarin-9-one showed that in polar solvents (methanol) it prefers the enol form. Data of spectral measurements were compared with results of semiempirical (MNDO, AM1, and PM3) and nonempirical quantum-chemical calculations (with 3-21G, 6-31G*, and 31G** basis sets).
Similar content being viewed by others
REFERENCES
Traven', V.F., Manaev, A.V., Safonova, O.B., Chibisova, T.A., Lysenko, K.A., and Antipin, M.Yu., Zh. Obshch. Khim., 2000, vol. 70, no. 5, pp. 853-864.
Dall'Acqua, F., Vedaldi, D., Caffieri, S, Guiotto, A., Rodighiero, P., Carrlassare, F., and Bordin F., J. Med. Chem., 1981, vol. 24, no. 2, pp. 178-184.
Guiotto, A., Rodighiero, P., Manzini, P., Pfstorini, G., Carlassare, F., Vedaldi, D., Dall'Acqua, F., Tamaro, M., Rechia, G., and Cristofolini, M., J. Med. Chem., 1984, vol. 27, no. 8, pp. 959-966.
Traven, V.F., Kravtchenko, D.V., and Chibisova, T.A., Mendeleev Commun., 1995, no. 1,p p. 21-22.
Traven, V.F., Kravtchenko, D.V., Chibisova, T.A., Shorshnev, S.V., Eliason, R., and Wakefield, D.H., Heterocycl. Commun., 1996, vol. 2, no. 4, pp. 345-354.
Traven, V.F., Kravtchenko, D.V., and Chibisova, T.A., Mendeleev Commun., 1997, no. 7, pp. 249-250.
Kravtchenko, D.V., Chibisova, T.A., and Traven, V.F., Heterocycl. Commun., 1997, vol. 3, no. 4, pp. 331-338.
Traven, V.F., Vorobjeva, L.I., Chibisova, T.A., Carberry, Ed.A., and Beyer, N.J., Can. J. Chem., 1997, vol. 75, no. 3, pp. 365-376.
Griffiths, J., Dyes Pigm., 1982, vol. 3, no. 3, pp. 211-233.
Kravchenko, D.V., Chibisova, T.A., Traven', V.F., Zh. Org. Khim., 1999, vol. 35, no. 6, pp. 924-935.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F., and Stewart, J.J.P., J. Am. Chem. Soc., 1985, vol. 107, no. 13, pp. 3902-3909.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Gill, P.M.W., Johnson, B.G., Robb, M.A., Cheeseman, J.R., Keith, T., Petersson, G.A., Montgomery, J.A., Raghvachari, K., Al-Laham, M.A., Zakrzewski, V. G., Ortiz, J.V., Foresman, J.B., Ciosloski, J., Stefanov, B.B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y., Chen, W., Wong, M.W., Andres, J.L., Reploge, E.S., Gomperts, R., Martin, R.L., Fox, D.J., Binkley, J.S., Defrees, D.J., Baker, J., Stewart, J.P., Head-Gordon, M., Gonzalez, C., and Pople, J.A., GAUSSIAN 94, Revision D.1., Pittsburg: Gaussian, 1995.
Hehre, W.J., Radom, L., Schleyer, P.v.R., and Pople, J., Ab Initio Molecular Orbital Theory, New York: Wiley, 1986.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Safonova, O.B., Kravchenko, D.V., Senchenya, I.N. et al. Electronic Structure of π Systems: XIX. Keto-Enol Tautomerism of Dihydrofurocoumarinones. Russian Journal of General Chemistry 71, 546–552 (2001). https://doi.org/10.1023/A:1012331118017
Issue Date:
DOI: https://doi.org/10.1023/A:1012331118017