Abstract
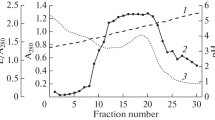
A new method for isolation of leukocyte serine proteinases has been developed. Elastase (EC 3.4.21.37) and cathepsin G (EC 3.4.21.20) have been isolated from dog neutrophils and purified to homogeneous state. The results of inhibitor analysis indicate that the enzymes belong to the group of serine proteinases. Some physical and chemical characteristics of the purified enzymes have been determined. The molecular weights of the enzymes are 24.5-26 kD for the elastase and 23.5-25.5 kD for the cathepsin G. The cathepsin G is a glycoprotein, while the elastase molecule lacks carbohydrate components. The cathepsin G exhibits a broad pH optimum of catalytic activity in the range of 7.0-9.0; the pH optimum for the elastase is 8.0-8.5. The Michaelis constant of the elastase for N-t-Boc-L-alanine p-nitrophenyl ester is 0.10 mM; the Michaelis constant of the cathepsin G for N-benzoyl-L-tyrosine ethyl ester is 0.42 mM.
Similar content being viewed by others
REFERENCES
Kokryakov, V. N. (1990) Vopr.Med.Khim., 6, 13–16.
Kokryakov, V. N. (1999) Biology of Animal Antibiotics [in Russian], Nauka, St. Petersburg.
Lehrer, R. I., Ganz, T., and Selsted, M. E. (1988) Ann.Int.Med., 109, 127–142.
Gabay, J. E., and Almeida, R. P. (1993) Curr.Opin.Immunol., 5, 97–102.
Levy, O. (1996) Eur.J.Haematol., 56, 263–277.
Garcia, R., Gusmani, L., Murgia, R., Guarnaccia, C., Cinco, M., and Rottini, G. (1998) Infection Immunity, 6, 1408–1412.
Miyasaki, K. T., Bodeau, A. L., Pohl, J., and Shafer, W. M. (1993) Antimicrobial Agents Chemotherapy, 37, 2710–2715.
Shafer, W. M., Shepherd, M. E., Boltin, B., Wells, L., and Pohl, J. (1993) Infection Immunity, 61, 1900–1908.
Chertov, O., Michiel, D. F., Xu, L., Wang, J. M., Tani, K., Murphy, W. J., Longo, D. L., Taub, D. D., and Oppenheim, J. J. (1996) J.Biol.Chem., 271, 2935–2940.
Klickstein, L. B., Kaempfer, C. E., and Weintraub, B. U. (1982) J.Biol.Chem., 257, 1504–1506.
Si-Tahar, M., Renesto, P., Falet, H., Rendu, F., and Chignard, M. (1996) Biochem.J., 313, 401–408.
Bjork, P., Axcelsson, L., and Ohlsson, K. (1991) Biol.Chem.Hoppe-Seyler., 372, 419–426.
Delshammar, M., and Ohlsson, K. (1976) Eur.J.Biochem., 69, 125–131.
Ohlsson, K. (1978) in Neutral Proteases of Human Polymorphonuclear Leukocytes(Havemann, K., and Janoff, A., eds.) Baltimore, Munich, pp. 89–101.
Desser, R. K., Himmelhoch, S. R., Evans, W. H., Januska, M., Mage, M., and Shelton, E. (1972) Arch.Biochem.Biophys., 148, 452–465.
Panyim, S., and Chalkley, R. (1969) Arch.Biochem.Biophys., 130, 337–346.
Dawson, M., Elliott, D., Elliott, W., and Jones, K. (1991) Data for Biochemical Research [Russian translation], Mir, Moscow.
Schagger, H., and von Jagow, G. (1987) Analyt.Biochem., 166, 368–379.
Visser, L., and Blaut, E. (1972) Biochim.Biophys.Acta, 268, 257–260.
Hummel, B. C. W. (1959) Canad.J.Biochem.Physiol., 37, 1393–1399.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951) J.Biol.Chem., 193, 265–275.
Janoff, A., and Feinstein, G. (1978) in Neutral Proteases of Human Polymorphonuclear Leukocytes(Havemann, K., and Janoff, A., eds.) Baltimore, Munich, pp. 102–117.
Schmidt, W., and Havemann, K. (1978) in Neutral Proteases of Human Polymorphonuclear Leukocytes(Havemann, K., and Janoff, A., eds.) Baltimore, Munich, pp. 150–160.
Sinha, S., Watorek, W., Karr, S., Giles, J., Bode, W., and Travis, J. (1987) Proc.Natl.Acad.Sci.USA, 84, 2228–2232.
Taylor, J. C., and Crawford, I. P. (1975) Arch.Biochem.Biophys., 169, 91–101.
Kraeva, L. N., Kokryakov, V. N., Chesnokov, I. N., Yakovleva, M. F., and Lyzlova, S. N. (1988) Biokhimiya, 53, 655–662.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berlov, M.N., Lodygin, P.A., Andreeva, Y.V. et al. Isolation and Some Physical and Chemical Properties of Elastase and Cathepsin G from Dog Neutrophils. Biochemistry (Moscow) 66, 1008–1013 (2001). https://doi.org/10.1023/A:1012325810788
Issue Date:
DOI: https://doi.org/10.1023/A:1012325810788