Abstract
Purpose. The influence of oleic acid (OA) and phosphatidylethanolamine (PhE) as membrane constituents on the partition behavior of (RS)-[3H]propranolol between unilamellar liposomes and buffer was studied as a function of pH.
Methods. Partition studies were performed by means of equilibrium dialysis at 37°C over a broad pH range at a molar propranolol to lipid ratio in the membrane of 10−6.
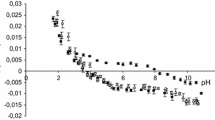
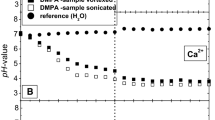
Results. As compared to the standard phosphatidylcholine (PhC)-liposome/buffer partition system PhE and OA have an enhancing effect on the apparent partition coefficient (D) of (RS)-[3H]propranolol between pH 6 and 11. Data analysis with Henderson-Hasselbalch equations revealed that the neutral propranolol has a higher affinity to membranes containing net neutral charged PhE than to pure PhC-liposomes. Net negatively charged PhE seems to have no significant influence on the partitioning. Deprotonated OA caused an increase in the true partition coefficient (P) of the protonated propranolol. Neutral OA showed no influence on the partitioning. From the fit D vs pH curves and from zeta potential measurements of the liposomes the intrinsic pKa values of the membranous lipids were calculated as 7.5 to 7.8 for OA and 9.7 to 9.8 for PhE.
Conclusions. Since the pKa of membranous OA is close to the physiological pH and D depends on the ionisation state of OA, small pH changes around the physiological pH may cause large differences in drug-lipid membrane interactions.
Similar content being viewed by others
REFERENCES
W. Nernst. Z. Phys. Chem. 8:110–139 (1891).
C. Hansch, A. Leo, and D. Hoekman. Exploring QSAR. Hydrophobic, Electronic, and Steric Constants. ACS Professional Reference Book. American Chemical Society, Washington, DC, 1995.
H. Miyoshi, H. Maeda, N. Tokutake, and T. Fujita. Bull. Chem. Soc. Jpn. 60:4357–4362 (1987).
C. J. Alcorn, R. J. Simpson, D. E. Leahy, and T. J. Peters. Biochem. Pharmacol. 45:1775–1782 (1993).
R. P. Austin, A. M. Davis, and C. N. Manners. J. Pharm. Sci. 84:1180–1183 (1995).
G. M. Pauletti and H. Wunderli-Allenspach. Eur. J. Pharm. Sci. 1:273–282 (1994).
C. Ottiger and H. Wunderli-Allenspach. Eur. J. Pharm. Sci., in press.
S. D. Krämer and H. Wunderli-Allenspach. Pharm. Res. 13:1851–1855 (1996).
H. Wunderli-Allenspach, M. Günthert, and S. Ott. Biochemistry 32:900–907 (1993).
M. J. Hope, M. B. Bally, G. Webb, and P. R. Cullis. Biochim Biophys. Acta. 812:55–65 (1985).
T. Teorell and E. Stenhagen. Biochem. Z. 299:416–419 (1938).
M. Takayama, S. Itoh, T. Nagasaki, and I. Tanimizu. Clin. Chim. Acta 79:93–98 (1977).
F. C. Tsui, D. M., Ojcius, and W. L. Hubbell. Biophys. J. 49:459–468 (1986).
R. Lieckfeldt, J. Villalaín, J. Gómez-Fernández, and G. Lee. Pharm. Res. 12:1614–1617 (1995).
A. Spector. J. Lipid Res. 16:165–179 (1975).
J. Miyazaki, K. Hideg, and D. Marsh. Biochim. Biophys. Acta 1103:62–68 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Krämer, S.D., Jakits-Deiser, C. & Wunderli-Allenspach, H. Free Fatty Acids Cause pH-Dependent Changes in Drug-Lipid Membrane Interactions Around Physiological pH. Pharm Res 14, 827–832 (1997). https://doi.org/10.1023/A:1012175111401
Issue Date:
DOI: https://doi.org/10.1023/A:1012175111401