Abstract
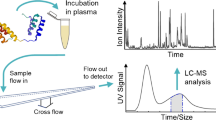
Purpose. The applicability of Asymmetrical Flow Field-Flow Fractionation (Asymmetrical Flow FFF) as an alternative tool to examine the distribution of a lipophilic drug (N-Benzoyl-staurosporine) within human plasma protein fractions was investigated with respect to high separation speed and loss of material on surfaces due to adsorption.
Methods. Field-Flow Fractionation is defined as a group of pseudo-chromatographic separation methods, where compounds are separated under the influence of an externally applied force based on differences in their physicochemical properties. This method was used to separate human plasma in its protein fractions. The drug distribution in the fractions was investigated by monitoring the fractionated eluate for drug content by fluorescence spectroscopy.
Results. Human plasma was separated into human serum albumin (HSA), high density lipoprotein (HDL), α2-macroglobulin and low density lipoprotein (LDL) fractions in less than ten minutes. Calibration of the system and identification of the individual fractions was performed using commercially available protein reference standards. The influence of membrane type and carrier solution composition on the absolute recovery of N-Benzoyl-staurosporine and fluorescein-isothio-cyanate-albumin (FITC-albumin) was found to be quite significant. Both factors were optimized during the course of the investigations. N-Benzoyl-staurosporine was found to be enriched in the fraction containing HSA.
Conclusions. If experimental conditions are thoroughly selected and controlled to suppress drug and plasma protein adsorption at the separation membrane, Asymmetrical Flow FFF shows high recoveries and fast separation of human plasma proteins, and can be a reliable tool to characterize drug / plasma protein interactions. For analytical purposes it has the potential to rival established technologies like ultracentrifugation in terms of ease-of-use, precision, and separation time.
Similar content being viewed by others
REFERENCES
J.-P. Tillement, G. Houin, R. Zini, S. Urien, E. Albengres, J. Barré, M. Lecomte, P. D'Athis, and B. Sebille. Adv. Drug Res. 13:59–94 (1984).
R. J. Tallarida, R. B. Raffa, and P. McGonigle. Principles in General Pharmacology, Springer Verlag, New York, 1988, pp. 43–45.
F. Hervé, S. Urien, E. Albengres, J.-C. Duché, and J.-P. Tillement. Clin. Pharmacokin. Concepts. 26:44–58 (1994).
T. Tokui, C. Kuroiwa, S. Muramatsu, Y. Tokui, K. Sasagawa, T. Ikeda, and T. Komai. Biopharm. Drug Dispos. 16:91–103 (1995).
M. I. Mackness and P. N. Durrington. Lipoprotein separation and analysis for clinical studies. In C. A. Converse and E. R. Skinner (eds.), Lipoprotein Analysis, Oxford University Press, New York, 1992, pp. 1–39.
G. L. Mills, P. A. Lane, and P. K. Weech. A Guidebook to Lipoprotein Technique, Elsevier, Amsterdam, New York, Oxford, 1984, pp. 94–98.
L. L. Rudel, C. A. Marzetta, and F. L. Johnson. Separation and analysis of lipoproteins by gel filtration. In J. J. Albers and J. P. Segrest (eds.), Methods in Enzymology, Volume 129, Plasma Lipoproteins, Part B, Characterization, Cell Biology and Metabolism, Academic Press, Inc., Orlando, Florida, 1986, pp. 45–57.
J. C. Giddings, F. J. Yang, and M. N. Myers. Anal. Chem. 81:395–407 (1977).
J. C. Giddings, M. A. Benincasa, M.-K. Liu, and P. Liu. Liquid Chromatogr. 15:1729–1747 (1992).
J. C. Giddings, Science. 260:1456–1465 (1993).
P. Li and J. C. Giddings. J. Pharm. Sci. 85:895–898 (1995).
K. D. Caldwell. Anal. Chem. 60:959A–971A (1988).
A. Litzén. Asymmetrical Flow Field-Flow Fractionation. Doctoral thesis. University of Uppsala, Sweden, 1992
A. Litzén, J. K. Walter, H. Krischollek, and K.-G. Wahlund. Analytical Biochemistry. 212:469–480 (1993).
A. Litzén and K.-G. Wahlund. Chromatogr. 548:393–406 (1991).
K. G. Wahlund and A. Litzén. Chromatogr. 461:73–87 (1989).
T. A. Horbett. Adsorption of proteins and peptides at interfaces. In T. J. Ahem and M. C. Manning (eds.) Stability of Protein Pharmaceuticals. Part A: Chemical and Physical Pathways of Protein Degradation, Plenum Press, New York and London, 1992, pp. 195–214.
F. W. Putnam. Alpha, Beta, Gamma, Omega—The Roster of the Plasma Proteins. In F. W. Putnam (ed.) The Plasma Proteins, Structure, Function and Genetic Control, Academic Press, New York/San Francisco, London, 1975, p. 61.
M. J. Chapman. Comparative analysis of mammalian lipoproteins. In J. P. Segrest and J. J. Albers (eds.) Methods in Enzymology Volume 128, Plasma Lipoproteins, Part A, Preparation, Structure, and Molecular Biology, Academic Press, Inc., Orlando, Florida, 1986, pp. 70–144.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Madörin, M., van Hoogevest, P., Hilfiker, R. et al. Analysis of Drug/Plasma Protein Interactions by Means of Asymmetrical Flow Field-Flow Fractionation. Pharm Res 14, 1706–1712 (1997). https://doi.org/10.1023/A:1012171511285
Issue Date:
DOI: https://doi.org/10.1023/A:1012171511285