Abstract
Purpose. The genetic stability of a recombinant human factor VIII (rhFVIII) product expressed in Chinese hamster ovary cells (Recombinate™) has been evaluated through comparisons of the protein produced at the beginning, middle and end of a typical production campaign.
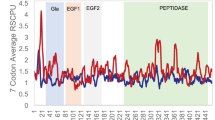
Methods. Recombinant human factor VIII was incubated with thrombin, the resulting four polypeptides were isolated by RP-HPLC, subjected to proteolysis with trypsin, and the peptide mixtures were resolved by RP-HPLC. Tryptic peptide mixtures were subjected to online mass spectrometric analysis using an electrospray ionization source interfaced to a quadrupole mass analyzer scanning from 1950−200 amu, and the peptide ion data were compared for three lots produced from the beginning, middle and end of a production campaign.
Results. The UV elution profiles for each of the rhFVIIIa polypeptides were highly similar for factor VIII isolated from the beginning, middle and end of production. Total ion data from the peptide maps derived from three lots of rhFVIII were compared by MH1+ values as a function of scan range. A total of 918 ions were analyzed for the four polypeptides of rhFVIII produced at the beginning, middle and end of a production campaign. The ions were detected at the same relative retention times, as indicated by the similar scan numbers for the three lots.
Conclusions. These observations support that rhFVIII preparations produced from the beginning, middle and end of a production campaign were highly similar, and demonstrate genetic stability in the manufacturing process of Recombinate™.
Similar content being viewed by others
REFERENCES
W. K. Kane and E. W. Davie. Blood 71:539–555 (1988).
R. J. Kaufman. Annual Review of Medicine 43:325–339 (1992).
G. C. White, 2nd, C. W. McMillan, H. S. Kingdon, and C. B. Shoemaker. New England Journal of Medicine 320:166–170 (1989).
I. Scharrer. Annals of Hematology 63:172–176 (1991).
P. A. Foster and T. S. Zimmerman. Blood Reviews 3:180–191 (1989).
D. D. Pittman and R. J. Kaufman. Proc. Natl. Acad. Sci. USA 85:2429–2433 (1988).
D. Eaton, H. Rodriguez, and G. A. Vehar. Biochemistry 25:505–512 (1986).
G. Kemball-Cook, J. E. Tubbs, N. J. Dawson, and T. W. Barrowcliffe. British J. Haematol. 84:273–278 (1993).
E. Gomperts, R. Lundblad, and R. Adamson. Transfusion Medicine Reviews 6:247–251 (1992).
R. J. Kaufman, L. C. Wasley, and A. J. Dorner. Journal of Biological Chemistry 263:6352–6362 (1988).
H. M. Koplove. Annals of Hematology 68:S15–S20 (1994).
C. Henry. Analytical Chemistry 68:684A–677A (1996).
R. Adamson. Annals of Hematology 68:S9–S14 (1994).
M. M. Federici. Biologicals 22:151–159 (1994).
N. Burns. Biologicals 21:145–146 (1993).
K. B. Seamon. Biologicals 21:153–154 (1993).
W. Berthold. Biologicals 21:95–100 (1993).
M. E. Wiebe and N. S. Lin. Regulatory Practice for Biopharmaceutical Production (Lubiniecki, A S, Vargo, S A, eds.), pp. 33–62 (1994).
R. L. Garnick. Development of Biological Standards 76:117–130 (1992).
R. L. Garnick, N. J. Solli, and P. A. Papa. Analytical Chemistry 60:2546–2557 (1988).
A. L. Burlingame, R. K. Boyd, and S. J. Gaskell. Analytical Chemistry 68:599R–651R (1996).
S. A. Carr, M. E. Hemling, M. F. Bean, and G. D. Roberts. Analytical Chemistry 63:2802–2824 (1991).
J. J. Dougherty, Jr., L. M. Snyder, R. L. Sinclair, and R. H. Robins. Analytical Biochemistry 190:7–20 (1990).
V. Ling, A. W. Guzzetta, E. Canova-Davis, J. T. Stults, W. S. Hancock, T. R. Covey, and B. I. Shushan. Analytical Chemistry 63:2909–2915 (1991).
G. F. Lee and D. C. Anderson. Bioconjugate Chemistry 2:367–374 (1991).
S. A. Charman, L. E. McCrossin, and W. N. Charman. Pharmaceutical Research 10:1471–1477 (1993).
K. Biemann. Methods in Enzymology 193:351–360 (1990).
J. V. O'Connor. Biologicals 21:111–117 (1993).
J. R. Whitaker and P. E. Graum. Analytical Biochemistry 106:156–159 (1980).
D. E. Garfin. Methods in Enzymology 182:425–441 (1990).
R. J. Harris, A. A. Murnane, S. L. Utter, K. L. Wagner, E. T. Cox, G. D. Polastri, J. C. Helder, and M. B. Sliwkowski. Biol Technology 11:1293–1297 (1993).
J. A. Schrier, R. A. Kenley, R. Williams, R. J. Corcoran, Y. Kim, R. P. Northey, Jr., D. D'Augusta, and M. Huberty. Pharmaceutical Research 10:933–944 (1993).
A. W. Guzzetta, L. J. Basa, W. S. Hancock, B. A. Keyt, and W. F. Bennett. Analytical Chemistry 65:2953–2962 (1993).
G. C. Davis, H. A. Havel, P. M. Kovach, R. M. Riggin, J. K. Towns, D. P. Allen, and J. J. L'Italien. Regulatory Practice for Biopharmaceutical Production (Lubiniecki, A S, Vargo, S A, eds.), pp. 87–146 (1994).
G. D. Roberts, W. P. Johnson, S. Burman, K. R. Anumula, and S. A. Carr. Analytical Chemistry 67:3613–3625 (1995).
S. A. Carr, M. J. Huddleston, and R. S. Annan. Analytical Biochemistry 239:180–192 (1996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Besman, M.J., Shiba, D. Evaluation of Genetic Stability of Recombinant Human Factor VIII by Peptide Mapping and On-line Mass Spectrometric Analysis. Pharm Res 14, 1092–1098 (1997). https://doi.org/10.1023/A:1012169832299
Issue Date:
DOI: https://doi.org/10.1023/A:1012169832299