Abstract
Purpose. The purpose of this study was to develop a new ternary polymeric matrix system that is easy to manufacture and that delivers a highly soluble drug over long periods of time.
Methods. Pectin, hydroxypropylmethylcellulose (HPMC), and diltiazem HC1 granulated with gelatin at optimized ratios were blended at different loading doses and directly compressed. Swelling behavior, dissolution profiles and the effect of hydrodynamic stress on release kinetics were evaluated.
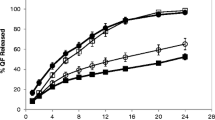
Results. Diltiazem release kinetics from the ternary polymeric system was dependent on the different swelling behavior of the polymers and varied with the drug loading dose and hydrodynamic conditions. Drug release followed either non-Fickian or Case II transport kinetics. The relative influence of diffusion and relaxational/dissolution effects on release profiles for different drug loadings was calculated by a nonlinear regression approach. Photographs taken during swelling show that the anisotropic nature of the gel structure, drug loading dose, swelling capacity of polymers used, and the design of delivery system all play important roles in controlling the drug release and dissolution/ erosion processes.
Conclusions. Zero-order delivery of diltiazem HC1 from a simple tablet matrix was achieved. The ternary polymeric system developed in this study is suitable for controlled release of highly soluble drugs. It offers a number of advantages over existing systems, including ease of manufacturing and of release modulation, as well as reproducibility of release profiles under well defined hydrodynamic conditions. Our delivery system has the potential to fully release its drug content in a controlled manner over a long time period and to dissolve completely.
Similar content being viewed by others
REFERENCES
United State Patent No. 4,839,177 (1989).
U. Conte, L. Maggi, and A. La Manna. S.T.P. Pharma. Sciences. 412:107-113 (1994).
K. Heilmann. Therapeutic Systems, Rate-Controlled Drug Delivery: Concept and Development, 2nd ed., Thieme-Stratton, New York, 50p 1984.
B. Eckenhoff, F. Theeuwes, and J. Urquhart. Pharm. Techn. 5:35-44 (1881).
L. Yang and R. Fassihi. J. Pharm. Sci. 85:170-173 (1996).
M. Sato, T. Nagao, I. Yamaguchi, H. Nakajima, and A. Kiyomoto. Arzneim.-Forsch. 21:1338-1343 (1971).
M. S. Smith, C. P. Verghese, D. G. Shand, and E. L. C. Pritchett. Am. J. Cardiol. 51:1369-1374 (1983).
L. M. Prisant, B. Bottini, J. T. Dipiro, and A. A. Carr. Am. J. Med. 93(suppl 2A):45S-55S (1992).
M. Gibaldi. Pharmaceutical News. 3:8-12 (1996).
J. G. Wagner. Drug. Stand. 28:30-34 (1960).
T. Higuchi. J. Pharm. Sci. 52:1145-1149 (1963).
A. W. Hixson, J. H. Crowell. Ind. Eng. Chem., 23:923-927 (1931).
G. Xu and H. Sunada. Chem. Pharm. Bull. 43:483-487 (1995).
H. Kim, A. R. Fassihi. J. Pharm. Sci. 86:316-322 (1997).
H. Kim, A. R. Fassihi. J. Pharm. Sci. 86:323-328 (1997).
S. C. Sutton, K. Engle, R. A. Deeken, K. E. Pluteand R. D. Shaffer. Pharm. Res. 8:84-87 (1991).
L. Yang and A. R. Fassihi. J. Control. Rel. 11:135-140 (1996).
R. T. C. Ju, P. R. Nixon, M. V. Patel, and M. Tong. Proceed. Intern. Symp. Control. Rel. Bioact. Mater. 22:59-60 (1995).
P. L. Ritger and N. A. Peppas. J. Cont. Rel. 5:37-42 (1987).
P. Colombo, R. Bettini, R.; G. Massimo, P. L. Catellani, P. Santi, and N. A. Peppas. J. Pharm. Sci. 84:991-997 (1995).
W. D. Lindner and B. C. Lippold. Pharm. Res. 12:1781-1785 (1995).
A. T. Pham and P. I. Lee. Pharm. Res. 11:1379-1384 (1994).
N. A. Peppas and J. J. Sahlin. Int. J. Pharm. 57:169-172 (1987).
A. R. Beren and H. B. Hopfenberg. Polymers. 19:489 (1978).
W. Sutananta, D. Q. M. Craig, and J. M. Newtons. J. Pharm. Pharmcol. 47:182-187 (1995).
D. L. Wolfram and C. L. Bernhard. Pharm. Res. 12:1781-1785 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, H., Fassihi, R. A New Ternary Polymeric Matrix System for Controlled Drug Delivery of Highly Soluble Drugs: I. Diltiazem Hydrochloride. Pharm Res 14, 1415–1421 (1997). https://doi.org/10.1023/A:1012124806316
Issue Date:
DOI: https://doi.org/10.1023/A:1012124806316