Abstract
Purpose. To investigate the feasibility of transdermal iontophoretic delivery of apomorphine in patients with Parkinson's disease, transdermal transport rates were optimized and validated across human stratum corneum and freshly dermatomed human skin in vitro.
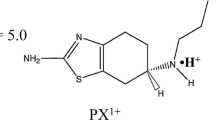
Methods. In all experiments R-apomorphine hydrochloride was applied in the anodal compartment. The effect on the flux of the following parameters was studied, using a flow through transport cell: current density, pH, concentration, ionic strength, osmolarity, buffer strength, temperature and skin type.
Results. Transdermal transport of apomorphine was directly controlled by the presence or absence of current. Passive delivery was minimal and no depot effect was observed. A linear relationship was found between current density and steady-state flux. At room temperature the lag time was 30 to 40 minutes. A maximal steady-state flux was obtained when the donor concentration approached maximum solubility. By increasing the temperature of the acceptor chamber to 37°C, the steady-state flux was increased by a factor of 2.3 and the lag time decreased to ± 3 minutes. No effect of osmolarity and buffer strength, and only a small effect of ionic strength and pH on the transport rate were observed. The flux through dermatomed human skin was decreased compared to stratum corneum. This effect was shown not to be caused by skin metabolism.
Conclusions. The results obtained in vitroindicate that the iontophoretic delivery of apomorphine can be controlled and manipulated accurately by the applied current. The in vitro flux furthermore depends on the donor composition, temperature and skin type. Under optimized conditions, transport rates resulting in therapeutically effective plasma concentrations are feasible, assuming a one to one in vitro/in vivo correlation.
Similar content being viewed by others
REFERENCES
M. Danhof, J. W. Mandema, A. Hoogerkamp, and R. A. Mathôt. Eur. J. Drug Metab. Pharmacokinet. 18:41–47 (1993).
R. J. Hardie, A. J. Lees, and G. M. Stern. Brain 107:487–50 (1984).
T. van Laar, R. van der Geest, M. Danhof, H. E. Boddé, P. H. Goossens, and R. A. C. Roos. Stepwise intravenous infusion of apomorphine to determine the therapeutic window in patients with Parkinson's disease. Clin. Neuropharmacol. (in press).
A. J. Lees. Fundam. Clin. Pharmacol. 7:121–128 (1993).
J. P. Frankel, A. J. Lees, P. A. Kempster, and G. M. Stern. J. Neurol. Neurosurg. Psychiatry 53:96–101 (1990).
S. T. Gancher, J. G. Nutt, and W. R. Woodward. Mov. Disord. 6:212–216 (1991).
F. Durif, E. Beyssac, F. Coudoré, M. Paire, A. Eschalier, M. Aiache, and J. Lavarenne. Clin. Neuropharmacol. 17:445–453 (1994).
B. H. Sage. Iontophoresis. In E. W. Smith and H. I. Maibach (eds.), Percutaneous Penetration Enhancers, CRC Press, Boca Raton, 1995, pp. 351–368.
N. H. Yoshida and M. S. Roberts. Adv. Dr. Del. Rev. 9:239–264 (1992).
P. G. Green. J. Control. Rel. 41:33–48 (1996).
R. van der Geest, M. Danhof, and H. E. Boddé. Validation and testing of a new iontophoretic continuous flow through transport cell. J. Control. Rel. (1997) in press.
T. van Laar, J. J. van Hilten, C. Neef, A. W. F. Rutgers, S. Pavel, and J. A. Bruyn. Allergic reactions to subcutaneous infusion of apomorphine in patients with Parkinson's disease are caused by EDTA: A histological study. (submitted).
R. van der Geest, P. Kruger, J. M. Gubbens-Stibbe, T. van Laar T, H. E. Boddé, and M. Danhof. Assay of R-apomorphine, S-apomorphine, apocodeine and isoapocodeine and their glucuronide and sulphate conjugates in plasma and urine of patients with Parkinson's disease. J. Chromatogr. (1997) in press.
P. G. Green, R. S. Hinz, A. Kim, F. C. Szoka, and R. H. Guy. Pharm. Res. 8:1121–1127 (1991).
N. M. Volpato, P. Santi, and P. Colombo. Pharm. Res. 12:1623–1627 (1995).
V. Préat and S. Thysman. Int. J. Pharm. 96:189–196 (1993).
D. F. Hager, M. J. Laubach, J. W. Sharkey, and J. R. J. Control. Rel. 23:175–182 (1993).
R. van der Geest, T. van Laar, P. P. Kruger, J. M. Gubbens-Stibbe, H. E. Boddé, R. A. C. Roos, and M. Danhof. Pharmacokinetics, enantiomer interconversion and metabolism of R-apomorphine in patients with idiopathic Parkinson's disease. (submitted)
R. V. Padmanabhan, J. B. Phipps, G. A. Lattin, and R. J. Sawchuk. J. Control. Rel. 11:123–135 (1990).
M. B. Delgado-Charro and R. H. Guy. Pharm. Res. 11:929–935 (1994).
A. J. Hoogstraate, V. Srinivasan, S. M. Sims, and W. I. Higuchi. J. Control. Rel. 31:41–47 (1994).
J. E. Sanderson, S. Riel, and R. Dixon. J. Pharm. Sci. 78:361–364 (1989).
L. Wearley, J-C. Liu, and Y. W. Chien. J. Control. Rel. 8:237–250 (1989).
J. B. Phipps and J. R. Gyory. Adv. Dr. Del. Rev. 9:137–176 (1992).
I. H. Patel and R. H. Levy. J. Pharmacokin. Biopharm. 2:337–346 (1974).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Geest, R., Danhof, M. & Boddé, H.E. lontophoretic Delivery of Apomorphine I: In Vitro Optimization and Validation. Pharm Res 14, 1798–1803 (1997). https://doi.org/10.1023/A:1012100417645
Issue Date:
DOI: https://doi.org/10.1023/A:1012100417645