Abstract
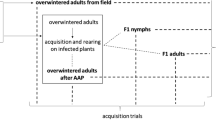
A study was carried out on the transmission parameters of the European stone fruit yellows phytoplasma by the vector Cacopsylla pruni. In the greenhouse, using groups of psyllids, the minimum acquisition period was 2–4 days, the minimum latent period 2–3 weeks and the minimum inoculation period 1–2 days. The vectors retained infectivity until their death. Under natural conditions retention of infectivity in C. pruni lasts through the winter and the following spring, when the overwintering insects reach the stone fruit trees, they are already infected and infective. The research shows that the vector C. pruni transmits the European stone fruit yellows phytoplasma in a persistent manner.
Similar content being viewed by others
References
Alma A, Navone P, Visentin C, Arzone A and Bosco D (2000) Rilevamento di fitoplasmi di ‘Apple proliferation’ in Cacopsylla melanoneura (F¨orster) (Homoptera Psyllidae). Petria 10: 141-142
Carraro L, Loi N and Ermacora P (2001) The ‘life cycle’ of pear decline phytoplasma in the vector Cacopsylla pyri. Journal of Plant Pathology (in press)
Carraro L, Loi N, Ermacora P, Gregoris A and Osler R (1998a) Transmission of pear decline by using naturally infected Cacopsylla pyri. Acta Horticulturae 472: 665-668
Carraro L, Loi N, Ermacora P and Osler R (1998b) High tolerance of European plum varieties to plum leptonecrosis. European Journal of Plant Pathology 104: 141-145
Carraro L, Loi N, Gregoris A, Ermacora P and Osler R (1996) Studies on the transmission of a phytoplasma from Chrysanthemum leucanthemum L. by the leafhopper Euscelidius variegatus Kbm. IOM Letters 4: 127-128
Carraro L, Osler R, Loi N, Ermacora P and Refatti E (1998c) Transmission of European stone fruit yellows phytoplasma by Cacopsylla pruni. Journal of Plant Pathology 80: 233-239
Chiykowski LN (1981) Epidemiology of diseases caused by leafhopper-borne pathogens. In: Maramorosch K and Harris KF (eds) Plant Diseases and Vector: Ecology and Epidemiology (pp 105-159), Academic Press, New York/London
Chiykowski LN and Sinha RC (1969) Comparative efficiency of transmission of aster yellows by Elymana virescens and Macrosteles fascifrons and the relative concentration of the causal agent in the vectors. Journal of Economic Entomology 62: 883-886
Conci C, Rapisarda C and Tamanini L (1992) Annotated catalogue of Italian Psylloideae. Atti dell'Accademia Roveretana degli Agiati II-B (ser. VII): 104-107
Cousin MT, Moreau JP and Grison C (1968) Mise en évidence de discontinutés dans la transmission de la phyllodie du trèfle par Euscelis plebejus Fall. Annales des Epiphyties 9: 115-120
Davies DL, Clark MF and Adams AN (1998) The epidemiology of pear decline in the UK. Acta Horticulturae 472: 669-672
Doyle JJ and Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12: 13-15
Frisinghelli C, Delaiti L, Grando MS, Forti D and Vindimian ME (2000) Cacopsylla costalis (Flor, 1861), as a vector of apple proliferation in Trentino. Journal of Phytopathology 148: 425-431
Golino DA, Oldfield GN and Gumpf DJ (1987) Transmission characteristics of beet leafhopper transmitted virescence agent. Phytopathology 77: 954-957
Jensen DD, Griggs WH, Gonzales CQ and Schneider H (1964) Pear decline virus transmission by pear psylla. Phytopathology 54: 1346-1351
Legrand AI and Power AG (1994) Inoculation and acquisition of maize bushy stunt mycoplasma by its leafhopper vector Dalbulus maidis. Annals of applied Biology 125: 115-122
Lemoine J (1991) Deperissement du poirier: role de Psylla pyri dans sa dissemination. Arboriculture Fruitière 442: 28-32
Lorenz KH, Schneider B, Ahrens U and Seemüller E (1995) Detection of apple proliferation and pear decline phytoplasmas by PCR amplification of ribosomal and nonribosomal DNA. Phytopathology 85: 771-776
Malisano G, Firrao G and Locci R (1996) 16S rDNA-derived oligonucleotide probes for the differential diagnosis of plum leptonecrosis and apple proliferation. EPPO Bullettin 26: 421-428
Purcell AH (1979) Transmission of X-disease agent by the leafhoppers Scaphytopius nitridus and Acinopterus angulatus. Plant Disease Reporter 63: 49-552
Purcell AH (1982) Insect vector relationships with prokaryotic plant pathogens. Annual Review of Phytopathology 20: 397-417
Seemüller E, Marcone C, Lauer U, Ragozzino A and Göschl M (1998) Current status of molecular classification of the phytoplasmas. Journal of Plant Pathology 80: 3-26
Sinha RC (1984) Transmission mechanisms of mycoplasmalike organisms by leafhopper vectors. In: Harris KF (ed) Current Topics in Vector Research. Vol 2 (pp 93-109), Praeger Scientific, New York
Tsai JH (1979) Vector transmission of mycoplasmal agents of plant diseases. In: Whitcomb RF and Tully JD (eds) The Mycoplasmas. Vol III (pp 265-307), Academic Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carraro, L., Loi, N. & Ermacora, P. Transmission Characteristics of the European Stone Fruit Yellows Phytoplasma and its Vector Cacopsylla Pruni. European Journal of Plant Pathology 107, 695–700 (2001). https://doi.org/10.1023/A:1011923801387
Issue Date:
DOI: https://doi.org/10.1023/A:1011923801387