Abstract
Purpose. The purpose of this study was to characterize the conformation, aggregation, and stability of leuprolide on gelation.
Methods. Infrared spectra (FTIR) of leuprolide solutions and gels were collected in water, propylene glycol (PG), dimethyl sulfoxide (DMSO), and trifluoroethanol (TFE). Leuprolide solution and gel stability data were obtained by SEC and RP-HPLC.
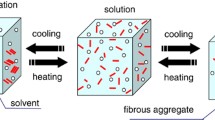
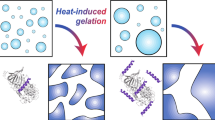
Results. Leuprolide was induced to gel with increasing peptide concentration, introduction of salts, and gentle agitation. Leuprolide dissolved in water (400 mg/ml) demonstrated FTIR spectra consisting of two major bands of equal intensity at 1615 cm−1 and 1630 cm−1, similar to inter- and intra-molecular β-sheet structure in proteins. When samples were gently agitated for 24 hours at 25°C, the formulation was observed to change from a viscous liquid to an opaque gel with a concomitant shift in infrared spectra from the equal intensity bands to mostly 1630 cm−1, indicating a shift to a preferred β-sheet structure. Incubation of leuprolide with 20−200 mM salts at 25°C and 37°C also produced gels ranging from clear to cloudy and stringy white precipitates. The gel and precipitate were marked by a shift of the predominant p-sheet band to 1630 cm−1 and 1615 cm−1, respectively. Leuprolide was also observed to gel and/or precipitate in mixtures of water, PG or TFE, but not in DMSO.
Conclusions. Birefringence was noted in many of the firmer gels. Both solutions and gels demonstrated minimal dimer or trimer formation, with no larger order aggregates detected. The chemical stability profile of gelled leuprolide was similar to that of the non-gelled water formulation by RP-HPLC.
Similar content being viewed by others
REFERENCES
G. V. Nikiforovich and G. R. Marshall. Conformation-function relationships in LHRH analogs II. Conformations of LHRH peptide agonists and antagonists. Int. J. Pept. Protein Res. 42:181–193 (1993).
A. L. Adjei and L. Hsu. Leuprolide and other LHRH analogues. Y. J. Wang and R. Pearlman (eds.). Stability and Characterization of Protein and Peptide Drugs. Plenum Press, New York, 1993, pp. 159–199.
M. E. Powers, A. Adjei, M. Y. Fu Lu, and M. C. Manning. Solution behaviour of leuprolide acetate, an LHRH agonist, as determined by circular dichroism spectroscopy. Int. J. Pharm. 108:49–55 (1994).
M. F. Powell, J. Fleitman, L. M. Sanders, and V. C. Si. Peptide liquid crystals: Inverse correlation of kinetic formation and thermodynamic stability in aqueous solution. Pharm. Res. 11:1352–1354 (1994).
M. F. Powell, L. M. Sanders, A. Rogerson, and V. Si. Parenteral peptide formulations: Chemical and physical properties of native luteinizing hormone-releasing hormone (LHRH) and hydrophobic analogues in aqueous solution. Pharm. Res. 8:1258–1263 (1991).
N. H. Andersen and P. K. Hammen. A Conformation-preference/potency correlation for GnRH analogs: NMR evidence. Bioorg. Med. Chem. 5:263–266 (1991).
J. Nestor, T. Ho, R. A. Simpson, B. L. Hormer, G. H. Jones, G. I. McRae, and B. H. Vickery. Synthesis and biological activity of some very hydrophobic superagonist analogs of luteinizing hormone-releasing hormone. J. Med. Chem. 25:795 (1982).
K. E. Dionne, J. Peery, J. C. Wright, S. Lautenback, F. Landrau, S. Tao, J. Magruder, P. A. Johnson, M. Cukierski, G. Chen, and J. Brown. An osmotically driven implantable therapeutic system for the human delivery of peptides and proteins. Proceed. Intern. Symp. Contrl. Rel. Bioact. Mater. 23:271 (1996).
D. M. Byler and H. Susi. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 25:469–487 (1986).
A. Dong, S. J. Prestrelski, S. D. Allison, and J. F. Carpenter. Infrared spectroscopic studies of lyophilization and temperature induced protein aggregation. J. Pharm. Sci. 84:415–424 (1995).
P. I. Haris and D. Chapman. The conformational analysis of peptides using fourier transform IR spectroscopy. Biopolymers 37:251–263 (1995).
M. Jackson, H. H. Mantsch, and J. H. Spencer. Conformation of magainin-2 and related peptides in aqueous solution and membrane environments probed by fourier transform infrared spectroscopy. Biochemistry 31:1289–1293 (1992).
J. W. Brauner, R. Mendelsohn, and F. G. Prendergast. Attenuated total reflectance fourier transform infrared studies of the interaction of melittin, two fragments of melittin, and δ-hemolsin with phosphatidylcholines. Biochemistry 26:8151–8158 (1987).
Y. Kim, C. A. Rose, Y. Liu, Y. Ozaki, G. Datta, and A. T. Tu. FTIR and near infrared FT-raman studies of the secondary structure of insulinotropin in the solid state: α-helix to β-sheet conversion induced by phenol and/or by high shear force. J. Pharm. Sci. 83:1175–1180 (1994).
J. M. Hadden, D. Chapman, and D. C. Lee. A comparison of infrared spectra of proteins in solution and crystalline forms. Biochim. Biophys. Acta 1248:115–122 (1995).
K. D. Collins and M. W. Washabaugh. The hofmeister effect and the behavior of water at interfaces. Q. Rev. Biophys. 14:323–422 (1985).
P. Huang, A. Dong, and S. C. Winslow. Effects of dimethyl sulfoxide, glycerol and ethylene glycol on secondary structures of cytochrome C and lysozyme as observed by infrared spectroscopy. J. Pharm. Sci. 84:387–392 (1995).
C. H. Wang and S. Damadoran. Thermal gelation of globular proteins: influence of protein conformation on gel strength. J. Agric. Food Chem. 39:433–438 (1991).
A. R. Oyler, R. E. Naldi, J. R. Lloyd, D. A. Graden, C. J. Shaw, and M. L. Cotter. Characterization of the solution degradation products of histerelin, a gonadotropin releasing hormone (LH/RH) agonist. J. Pharm. Sci. 80:271–275 (1991).
J. Okada, T. Seo, F. Kasahara, K. Takeda, and S. Kondo. New degradation product of Des-Gly10-NH2-LH-RH-Ethylamide (fertirelin) in aqueous solution. J. Pharm. Sci. 80:167–170 (1991).
J. E. Matsuura and M. C. Manning. Heat-induced gel formation of β-lactoglobulin: A study on the secondary and tertiary structure as followed by circular dichroism. J. Agric. Food Chem. 42:1650–1656 (1994).
N. Matsudomi, D. Rector, and J. E. Kinsella. Gelation of bovine serum albumin and β-lactoglobulin: Effects of pH, salts and thiol reagents. Food Chemistry 40:55–69 (1991).
D. M. Mulvihill and J. E. Kinsella. Gelation of β-lactoglobulin: Effects of sodium chloride and calcium chloride on the rheological and structural properties of gels. J. Food Science 53:231–236 (1988).
M. Murata, F. Tani, T. Higasa, N. Kitabatake, and E. Doi. Heat-induced transparent gel formation of bovine serum albumin. Biosci. Biotech. Biochem. 57:43–46 (1993).
E. Doi and N. Kitabatake. Structure of glycinin and ovalbumin gels. Food Hydrocolloids 3:327–337 (1989).
E. Terzi, G. Holzemann, and J. Seelig. Self-association of β-amyloid peptide (1–40) in solution and binding to lipid membranes. J. Mol. Biol. 252:633–642 (1995).
S. Zhang, T. Holmes, C. Lockshin, and A. Rich. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc. Natl. Acad. Sci. USA 90:1343–1348 (1993).
B. Forood, E. Perez-Paya, R. A. Houghten, and S. E. Blondelle. Formation of an extremely stable polyalanine β-sheet macromolecule. Biochem. Biophys. Res. Commun. 211:7–13 (1995).
C. Soto and E. M. Castano. The conformation of Alzheimer's β-peptide determines the rate of amyloid formation and its resistance to proteolysis. Biochem. J. 314:701–707 (1996).
H. H. Bauer, M. Muller, J. Goette, H. P. Merkle, and U. P. Fringeli. Interfacial adsorption and aggregation associated changes in secondary structure of human calcitonin monitored by ATR-FTIR spectroscopy. Biochemistry 33:12276–12282 (1994).
I. Laczko, E. Vass, K. Soos, J. L. Varga, S. Szaraz, M. Hollosi, and B. Penke. Ca+2 and Al+3 induced conformational transitions of amyloid fragment H-Ile-Ile-Gly-Leu-Met-NH2. Arch. Biochem. Biophys. 335:381–387 (1996).
A. R. Salomon, K. J. Marcinowski, R. P. Friedland, and M. G. Zagorski. Nicotine inhibits amyloid formation by the β-peptide. Biochemistry 35:13568–13578 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tan, M.M., Corley, C.A. & Stevenson, C.L. Effect of Gelation on the Chemical Stability and Conformation of Leuprolide. Pharm Res 15, 1442–1448 (1998). https://doi.org/10.1023/A:1011914007940
Issue Date:
DOI: https://doi.org/10.1023/A:1011914007940