Abstract
Purpose. Ranitidine plasma concentration vs. time profiles and the extent of ranitidine absorption were examined in the presence and absence of pancreatico-biliary secretions in order to elucidate factors which may contribute to secondary peaks after oral ranitidine administration.
Methods. Ranitidine solution (300 mg) was administered to 4 fasting healthy subjects via an indwelling small-bore oroenteric tube located ∼16 cm distal to the pylorus. On 3 consecutive days, subjects randomly received ranitidine alone (control), ranitidine 10 min after 0.04 μg/kg IV cholecystokinin (CCK) sufficient to cause gall bladder emptying into the duodenum, and ranitidine 30 min after inflation of an occlusive duodenal balloon located ∼10 cm distal to the pylorus to prevent pancreatico-biliary secretions from reaching the dosing port or beyond. Small bowel transit time (SBTT; min) was measured by breath H2. Serial blood samples, obtained over 12 hours in each treatment, were analyzed by HPLC to determine ranitidine AUC0−2(ng*h/mL), as well as Cmax(ng/mL) and Tmax(min) of the first and subsequent peaks, if subsequent peaks were observed.
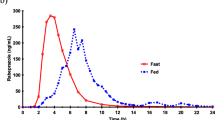
Results. Ranitidine AUC0−2and Cmax1, were not altered significantly by treatments; treatment effects on SBTT varied. Secondary peaks were observed in subjects #1 and #3 during the control treatment and subjects #2 and #4 during the CCK treatment. No secondary peaks were observed in any subject during the balloon treatment, and Tmax1was delayed.
Conclusions. Results support the hypothesis that pancreatico-biliary secretions (present in the intestinal lumen during control or CCK treatment) and gastrointestinal transit time may influence the occurrence of secondary peaks in ranitidine concentration vs. time profiles.
Similar content being viewed by others
REFERENCES
C. J. C. Roberts. Clinical pharmacokinetics of ranitidine. Clin. Pharmacokinet. 9:211-227 (1984).
C. Shim and J. Hong. Inter-and intrasubject variations in ranitidine pharmacokinetics after oral administration to normal male subjects. J. Pharm. Sci. 78:990-994 (1989).
E. F. Woodings, G. T. Dixon, C. Harrison, P. Carley, and D. A. Richards. Ranitidine-a new H2 receptor antagonist. Gut 21:187-191 (1980).
M. L. McFadyen, P. I. Folb, I. N. Marks, J. P. Wright, and W. Lucke. The pharmacokinetics of ranitidine in patients with chronic duodenal ulceration. Scan. J. Gastroenterol. 69(suppl):109-112 (1981).
S. S. Walkenstein, J. W. Dubb, W. C. Randolph, W. J. Westlake, R. M. Stote, and A. P. Intoccia. Bioavailability of cimetidine in man. Gastroenterology 74:360-365 (1979).
H. Kroemer and U. Klotz. Pharmacokinetics of famotidine in man. Intern. J. Clin. Pharmacol. Ther. Toxicol. 25:458-463 (1987).
S. Huang, T. B. Marriott, H. S. Weintraub, J. D. Arnold, J. Boccagno, R. Abels, and W. Harris. Clinical pharmacokinetics of etinidine. Biopharm. Drug Dispos. 9:477-86 (1988).
U. Klotz and S. Walker. Biliary excretion of H2-receptor antagonists. Eur. J. Clin. Pharmacol. 39:91-92 (1990).
A. B. Suttle and K. L. R. Brouwer. Bile flow but not enterohepatic recirculation influences the pharmacokinetics of ranitidine in the rat. Drug Metab. Dispos. 22:224-232 (1994).
A. Graham, C. von Bahr, B. Lindstrom, and A. Rosen. Bioavailability and pharmacokinetics of cimetidine. Eur. J. Clin. Pharmacol. 16:335-340 (1979).
Y. F. Hui, J. Kolars, Z. Hu, and D. Fleisher. Intestinal clearance of H2antagonists. Biochem. Pharmacol. 48:229-231 (1994).
D. C. Garg, D. J. Weidler, and F. N. Eshelman. Ranitidine bioavailability and kinetics in normal male subjects. Clin. Pharmacol. Ther. 33:445-452 (1983).
M. F. Williams, G. E. Dukes, W. Heizer, Y. H. Han, D. J. Hermann, T. Lampin, and L. J. Hak. Influence of gastrointestinal site of drug delivery on the absorption characteristics of ranitidine. Pharm. Res. 9:1190-1194 (1992).
T. Gramatte, E. El Desoky, and U. Klotz. Site-dependent small intestinal absorption of ranitidine. Eur. J. Clin. Pharmacol. 46:253-259 (1994).
A. B. Suttle and K. L. R. Brouwer. Regional gastrointestinal absorption of ranitidine in the rat. Pharm. Res. 12:1311-1315 (1995).
J. C. Bryson, G. E. Dukes, M. G. Kirby, W. D. Heizer, and J. R. Powell. Effect of altering small bowel transit time on sustained release theophylline absorption. J. Clin. Pharmacol. 29:733-738 (1989).
H. Korth, I. Muller, J. F. Erckenbrecht, and M. Wienbeck. Breath hydrogen as a test for gastrointestinal transit. Hepato-gastroenterol. 31:282-284 (1984).
J. H. Bond, Jr., and M. D. Levitt. Investigation of small bowel transit time in man utilizing pulmonary hydrogen (H2) measurements. J. Lab. Clin. Med. 85:546-555 (1975).
D. Farthing, K. L. R. Brouwer, I. Fakhry, and D. Sica. Solid phase extraction and determination of ranitidine in human plasma by a high performance liquid chromatographic method utilizing midbore chromatography. J. Liq. Chromatgr. Biomed. Appl. 688:350-353 (1997).
W. S. Fuchs, A. von Nieciecki, K. Molz, G. Popescu, A. Weil, M. F. Barkworth, S. Gay, A. Laicher, and F. Stanislaus. Effect of gallbladder contraction induced cholagogia on the pharmacokinetic profile of a sustained-release theophylline formulation. Arzneimittel-Forschung. 46:1120-1126 (1996).
S. D. Mithani, V. Bakatselou, C. N. TenHoor, and J. B. Dressman. Estimation of the increase in solubility of drugs as a function of bile salt concentration. Pharm. Res. 13:163-167 (1996).
K. Taniguchi, S. Muranishi, and H. Sezaki. Enhanced intestinal permeability to macromolecules. II. Improvement of the large intestinal absorption of heparin by lipid-surfactant mixed micelles in rat. Int. J. Pharm. 4:219-228 (1980).
S. Muranishi, N. Muranushi, and H. Sezaki. Improvement of absolute bioavailability of normally poorly absorbed drugs: inducement of the intestinal absorption of streptomycin and gentamycin by lipid-bile salt mixed micelles in rat and rabbit. Int. J. Pharm. 2:101-111 (1979).
T. Kimura, H. Sezaki and K. Kakemi. Effect of bile salts in the gastrointestinal absorption of drugs. IV: Site of intestinal absorption of sodium taurocholate and its consequence on drug absorption in the rat. Chem. Pharm. Bull 20:165-1662 (1972).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reynolds, K.S., Song, M.H., Heizer, W.D. et al. Effect of Pancreatico-Biliary Secretions and GI Transit Time on the Absorption and Pharmacokinetic Profile of Ranitidine in Humans. Pharm Res 15, 1281–1285 (1998). https://doi.org/10.1023/A:1011908412058
Issue Date:
DOI: https://doi.org/10.1023/A:1011908412058