Abstract
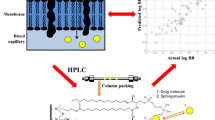
Purpose. The present study evaluates immobilized artificial membrane (IAM) chromatography for predicting drug permeability across the blood-brain barrier (BBB) and outlines the potential and limitations of IAMs as a predictive tool by comparison with conventional methods based on octanol/water partitioning and octadecylsilane (ODS)-HPLC.
Methods. IAM- and ODS-HPLC capacity factors were determined in order to derive the hydrophobic indices log kIAMand log kwfor two sets of compounds ranging from very lipid soluble (steroids) to more hydrophilic agents (biogenic amines). The uptake of the compounds across the in vivoBBB expressed as brain uptake index (BUI) has been correlated with these HPLC capacity factors as well as octanol/ water partition (ClogP) and distribution coefficients (log D7.4).
Results. For both test groups log kIAMcorrelates significantly with the respective log BUI of the drug (r2= 0.729 and 0.747, p < 0.05), whereas with log kw, log D7.4and ClogP there is only a correlation for the group of steroids (r2= 0.789, 0.659 and 0.809, p < 0.05) but not for the group of biogenic amines. There is a good correlation between log kIAMand log kw, ClogP or log D7.4for the group of steroids (r2= 0.945, 0.867 and 0.974, p < 0.01) but not for the biogenic amines.
Conclusions. All physico-chemical descriptors examined in this study equally well describe brain uptake of lipophilic compounds, while log kIAMis superior over log D7.4, ClogP and log kwwhen polar and ionizable compounds are included. The predictive value of IAMs combined with the power of HPLC holds thus great promise for the selection process of drug candidates with high brain penetration.
Similar content being viewed by others
REFERENCES
M. W. B. Bradbury. The blood-brain barrier. Exp. Physiol. 78:453-472 (1993).
V. A. Levin. Relation of octanol/water partition and molecular weight to rat brain capillary permeability J. Med. Chem. 23:682-684 (1980).
N. J. Abbott and I. A. Romero. Transporting therapeutics across the blood-brain barrier. Mol. Medicine Today 2:106-113 (1996).
R. A. Conradi, P. S. Burton, and R. T. Borchardt. Physico-chemical and biological factors that influence a drug's cellular permeability by passive diffusion. In V. Pliska. B. Testa and H. van de Waterbeemd (eds.), Lipophilicity in Drug Action and Toxicology, VCH, Weinheim, 1996. pp. 233-251.
C. A. Bailey, P. Bryla, and A. W. Malick. The use of the intestinal epithelial cell culture model, Caco-2, in pharmaceutical development. Adv. Drug Deliv. Rev. 22:85-100 (1996).
W. M. Pardridge. Transport of small molecules through the blood-brain barrier: biology and methodology. Adv. Drug Deliv. Rev. 15:5-36 (1995).
R. C. Young, R. C. Mitchell, T. H. Brown, C. R. Ganellin, R. Griffith, M. Jones, K. K. Rana, D. Saunders, I. R. Smith, N. E. Sore, and T. J. Wilks. Development of a new physicochemical model for brain penetration and its application to the design of centrally acting H2 receptor histamine antagonists. J. Med. Chem. 31, 656-671 (1988).
J. G. Dorsey and M. G. Khaledi. Hydrophobicity estimations by reversed-phase liquid chromatography. Implications for biological partitioning processes. J. Chrom. 656:485-499 (1993).
S. Ong. H. Liu, X. Qiu, G. Bhat, and C. Pidgeon. Membrane partition coefficients chromatographically measured using immobilized artificial membrane surfaces. Anal. Chem. 67:755-762 (1995).
M. H. Abraham and H. S. Chadha. Applications of a solvation equation to drug transport properties. In V. Pliska, B. Testa and H. van de Waterbeemd (eds.), Lipophilicity in Drug Action and Toxicology, VCH, Weinheim, 1996, pp. 311-337.
A. Seelig, R. Gottschlich, and R. M. Devant. A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proc. Natl. Acad. Sci USA 91:68-72 (1994).
S. C. Basak, B. D. Gute, and L. R. Drewes. Predicting blood-brain transport of drugs: a computational approach. Pharm. Res. 13:775-778 (1996).
F. M. Alvarez, C. B. Bottom, P. Chikhale, and C. Pidgeon. Immobilized artificial membrane chromatography. Prediction of drug transport across biological barriers. In T. Ngo (ed.), Molecular Interactions in Bioseparation, Plenum Press, New York, 1993, pp. 151-167.
C. Pidgeon, C. Marcus, and F. Alvarez. Immobilized artificial membrane chromatography: surface chemistry and application. In J. W. Kelly and T. O. Baldwin (eds.), Applications of Enzyme Biotechnology, Plenum Press, New York, 1991, pp. 201-220.
C. Y. Yang, S. J. Cai, H. Liu, and C. Pidgeon. Immobilized artificial membranes—screens for drug membrane interactions. Adv. Drug Del. Rev. 23:229-256 (1996).
C. Benistant, M. P. Dehouck, J. C. Fruchart, R. Cechelli, and M. Lagarde. Fatty acid composition of brain endothelial cells: effect of co-culture with astrocytes. J. Lipid Res. 36:2311-2319 (1995).
S. E. Wright, J. Courtland White, and L. Huang. Partitioning of tenoposide into membranes and the role of lipid composition. Biochim. Biophys. Acta—Biomembr. 1021:105-113 (1990).
Q. R. Smith. Methods of study. In M. W. B. Bradbury (ed.), Physiology and Pharmacology of the Blood-Brain BarrierSpringer Verlag, Heidelberg, 1992, pp. 23-52.
C. Hansch and A. Leo. Substituent constants for correlation analysis in chemistry and biology.Wiley and Sons, New York. pp. 169-330 (1979).
W. M. Pardridge and L. J. Mietus. Transport of steroid hormones throught the rat blood-brain barrier. J. Clin Invest. 64:145-154 (1979).
E. M. Cornford, L. D. Braun, W. H. Oldendorf, and M. A. Hill. Comparison of lipid-mediated blood-brain barrier penetrability in neonates and adults. Cell. Physiol. 12:C161-C168 (1982).
S. D. Kramer and H. Wunderli-Allenspach. The pH-dependence in the partitioning behaviour of (RS)-[3H]-propranolol between MDCK cell lipid vesicles and buffer. Pharm. Res. 13:1851-1855 (1996).
R. Kaliszan, A. Nasal, and A. Bucinski. Chromatographic hydrophobicity parameter determined on an immobilized artificial membrane column: relationships to standard measures of hydrophobicity and bioactivity. Eur. J. Med. Chem. 29:163-170 (1994).
M. H. Abraham, H. S. Chadha, R. A. E. Leitao, R. C. Mitchell, W. J. Lambert, R. Kaliszan, A. Nasal, and P. Habor. Determination of solute lipophilicity, as log P(octanol) and log P(alkane) using poly(styrene-divinylbenzene) and immobilized artificial membrane stationary phases in reversed-phase high-performance liquid chromatography. J. Chrom. A 766:35-47 (1997).
A. Reichel, A. Aleshaiker, D. J. Begley, and N. J. Abbott. In vitroscreening for drugs interacting with P-glycoprotein-mediated drug efflux using immortalised rat brain endothelial cells (RBE4). J. Physiol. 491:36P (1996).
A. Reichel, D. J. Begley, and N. J. Abbott. Structural requirements of phenylglycine derivatives for affinity to the L-system transporter at the blood-brain barrier. J. Physiol. 501:30P–31P (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reichel, A., Begley, D.J. Potential of Immobilized Artificial Membranes for Predicting Drug Penetration Across the Blood−Brain Barrier. Pharm Res 15, 1270–1274 (1998). https://doi.org/10.1023/A:1011904311149
Issue Date:
DOI: https://doi.org/10.1023/A:1011904311149