Abstract
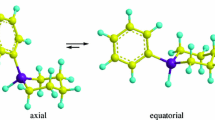
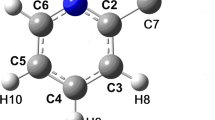
The X-ray diffraction study of the 2,2,5,5-tetramethyl-3,4-hexandione mono-hydrazone 1 shows a solid solution of two screwed conformers in the crystal. In each of these conformers, the conjugated C=O and C=N double bonds have an approximately perpendicular orientation with Φ = 101.1°(2) and −93.4°(2), respectively. AM1 theoretical calculations give the same result for the isolated molecule. The calculated rotational barrier around the central single bond of the conjugated moiety is about 45.98 kJ mol−1 which is higher than the classical values observed for 1,3 conjugated systems (28.42 kJ mol−1 in the 1,3-butadiene). Variable temperature 13C CPMAS NMR experiments show hindered rotation around the COC(CH3)3 tert-butyl group in the solid state. 1 crystallizes in the triclinic space group P 1 with a = 10.106(1)Å, b = 11.698(1)Å, c = 12.313(1)Å, α = 62.108(1)° β = 70.517(1)° γ = 66.052(1), V = 1157.0(3)Å3, D calc = 1.06 with Z = 4.
Similar content being viewed by others
References
Burgi, H.B.; Bartell, L.S. J. Am. Chem. Soc.,94,5236.
Wroczynski, R.J.; Mislow, K. J. Am. Chem. Soc.1979,101,3980.
Rithner, C.D.; Bushweller, C.H. J. Am. Chem. Soc.1985, 107,7823.
Anderson, J.E.; Kirsch, P.A.; Lomas, J.S. J. Chem. Soc. Chem. Commun.1988, 1065.
Kessler, H.; Gusowski, V.; Hanack, M. Tetrahedron lett.1968, 4665.
Stolow, R.D.; Marini, J.L. Tetrahedron lett.1971, 1449.
Viani, R.; Lapasset, J.; Aycard, J.P.; Bodot, H. J. Org. Chem.1979, 44,899 and references therein.
Lafrance, R.; Aycard, J.P.; Berger, J.; Bodot, H. Org. Magn. Res.1976, 8,95.
Viani, R.; Lapasset, J.; Aycard, J.P.; Lafrance, R.; Bodot, H. Acta Cryst.1978, B34,1190.
Trætteberg, M.; Hopf, H.; Lipka, H.; Hänel, R. Chem. Ber.1994, 127,1459.
Trætteberg, M.; Bakken, P.; Hopf, H.; Hänel, R. Chem. Ber.1994, 157,1469.
Newman, M.S.; Arkell, A. J. Org. Chem., 24,385.
Mackay, S.; Gilmore, C.J.; Edwards, C.; Treymane, M.; Stewart, N.; Shankland, K.; Maxus: A Computer Program for Solution and Refinement of Crystal Structure from Diffraction Data; University of Glasgow, Scotland, UK; Nonius BV, Delft, The Netherlands; MacScience Co. Ltd, Yokoama, Japan, 1998.
Nonius Kappa CCD Reference Manual.; Nonius BV, Delft, The Netherlands, 1998.
Dewar, M.S.J.; Zoebisch, E.G.; Healy, E.F.; Stawart, J.J.P. J. Am. Chem. Soc.1958, 107,3902.
Eliel, E.; Wilen, S.H. Stereochemistry of Organic Compounds; Wiley, New York, 1994.
Kalinowski, H.O.; Berger, S.; Braun, S. Carbon-13 NMR Spectroscopy; Wiley, Chichester, 1988.
Riddell, F.G.; Bernàth, G.; Fülöp, F. J. Am. Chem. Soc.1995, 117,2327.
Maverick, E.; Mirsky, K.; Knobler; Trueblood, K.N. Acta Crystallogr.1991, B47, 272.
Johnson, C.K. ORTEPII Report ORNL-5138; Oak Ridge National Laboratory, TN, 1976.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kakou-Yao, R., Pizzala, H., Pietri, N. et al. Solid-state conformational heterogeneity of the 2,2,5,5-tetramethyl-3,4-hexandione monohydrazone: X-ray diffraction and MAS NMR experiments. Journal of Chemical Crystallography 30, 593–598 (2000). https://doi.org/10.1023/A:1011310411157
Issue Date:
DOI: https://doi.org/10.1023/A:1011310411157