Abstract
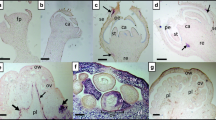
A pathogen was transmitted from apricot trees showing symptoms of viral infection to GF305 peach seedlings which reacted by stunting, shortened internodes and chlorotic mottling. The agent was transmitted to cherry, apricot, peach and plum by grafting and to several herbaceous hosts by mechanical inoculation. Isometric nepovirus-like particles of 30–31 nm diameter extracted from infected Chenopodium quinoa sedimented as two peaks in sucrose gradients. These particles contained two single stranded RNAs of approximately 5.9 and 7.9 kb, and a single coat protein subunit of 53.7 kDa. No cross-reactions were observed with a number of nepoviruses infecting fruit trees. Inoculation of purified particles to herbaceous or woody hosts reproduced the same symptoms caused by the original isolate. Sequencing of a 2.2 kbp cDNA clone covering the 3′ end of the small genomic RNA identified an open reading frame encoding a 317 aa N-truncated protein exhibiting significant similarities with the coat protein of nepoviruses. The 1257 nt long 3′ non-coding region showed up to about 65% homology to the equivalent region of members of the subgroup C of nepoviruses. The properties of this pathogen do not match those of any previously described nepovirus. It should therefore be considered as a new member of the subgroup C of nepoviruses, for which the name of Apricot latent ringspot virus (ALRSV) is proposed.
The nucleotide sequence reported in this work has been deposited in the EMBL databank under the accession number AJ278875.
Similar content being viewed by others
References
Bacher JW, Warkentin D and Ramsdell D, Hancock JF (1994) Selection versus recombination: what is maintaining identity in the 3′ termini of blueberry leaf mottle nepovirus RNA1 and RNA2? Journal of General Virology 75: 2133-2137
Boye R and Desvignes JC (1986) Biological techniques used for the study of new fruit virus disease. Acta Horticulturae 193: 261-268
Brunt AA, Crabtree K, Dallwitz MJ, Gibbs AJ, Watson L and Zurcher EJ (eds) (1996 onwards). Plant Viruses Online: Descriptions and Lists from the VIDE Database. Version: 16th January 1997. URL http://biology.anu.edu.au/Groups/ MES/vide/
Candresse T, Delbos RP, Le Gall O, Dunez J and Desvignes JC (1998) Characterisation of stocky prune virus, a newNepovirus detection in French prunes. Acta Horticulturae 472: 175-181
Damsteegt VD (1997) Prunus tomentosa as a diagnostic host for detection of plum pox virus and other Prunus viruses. Plant Disease 81: 329-332
Desvignes JC, Boye R, Cornaggia D and Grasseau N (1999)Virus diseases of fruit trees. Ed Ctifl, France
Doz B, Macquaire G, Delbos R and Dunez J (1980) Characterisation et role du RNA3, RNA satellite du virus des anneaux noirs de la tomate. Annales deVirologie (Institut Pasteur) 131E: 489-499
Dunez J, Delbos R, Desvignes JC, Marenaud C, Kuszala J and Vuittenez A(1971) Evidence of a ringspot type virus on Prunus cerasifera (myrobolan B). Annales de Phytopathologie Numaéro hors série, 117-128
Eveleigh ES and Allen WR (1982) Description of Longidorus diadecturus sp. (Nematoda: Longidoridae), a vector of the peach rosette mosaic virus in peach orchards in southwestern Ontario, Canada. Canadian Journal of Zoology 60: 112-115
Felsenstein J (1993) PHYLIP (Phylogeny Inference Package) version 3.5c. Distributed by the author. Department of Genetics, University of Washington, Seattle
Gentit P, Cornaggia D and Desvignes JC (1998) Identification and comparison of different Prunus phytoplasma diseases by indexing on GF305 peach seedlings in the greenhouse. Acta Horticulturae 472: 723-729
Gubler U and Hoffman BJ (1983) A simple and very efficient method for generating cDNA libraries. Gene 25: 263-269
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277: 680-685
Lammers AH, Allison RF and Ramsdell DC (1999) Cloning and sequencing of peach rosette mosaic virus RNA1. Virus Research 65: 57-73
Le Gall O, Candresse T and Dunez J (1995) A multiple alignment of the capsid protein sequences of nepoviruses and comoviruses suggests a common structure. Archives of Virology 140: 2041-2053
Martelli GP (1975) Some features of nematode-borne viruses and their relationships with the host plants. In: Lamberti F, Taylor CE and Seinhorst JW (eds) Nematode Vectors of Plant Viruses (pp 223-252) Plenum Press, London
Mayo MA and Robinson DJ (1996) Nepoviruses: molecular biology and replication. In: Harrison BD and Murant AF (eds) The PlantViruses: PolyhedralVirions and Bipartite RNAGenomes, Vol 5 (pp 139-184) Plenum Press, New York
Mayo MA, Barker H and Harrison BD (1982) Specificity and properties of the genome-linked proteins of nepoviruses. Journal of General Virology 59: 149-162
Nemeth M (1986) Virus, Mycoplasma and Rickettsia Diseases of Fruit Trees. Martinus Nijhoff, Dordrecht (NL)
Page RDM (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences 12: 357-358
Rott ME, Tremaine JH and Rochon DM (1991) Comparison of the 5 and 3 termini of tomato ringspot virus RNA1 and RNA2: evidence for RNA recombination. Virology 185: 468-472
Sambrook J, Fritsch EF and Maniatis T (1989) Molecular Cloning: A Laboratory Manual, 2nd edn, Cold Spring Harbour Laboratory, Cold Spring, New York
Sanfacon H (1995) Nepoviruses. In: Singh RP, Singh US and Kohmoto K (eds) Pathogenesis and Host Specificity in Plant Diseases, Viruses and Viroids, Vol 3 (pp 129-141) Pergamon Press, Oxford
Thompson JD, Higgins DG and Gibson TJ (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673-4680
Wellink J, Le Gall O, Sanfacon H, Ikegami M and Jones AT (2000) Family Comoviridae. In:Van Regenmortel MHV, Fauquet CM, Bishop DHL, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR and Wickner RB (eds) Family Comoviridae, in: Virus Taxonomy, Classification and Nomenclature of Viruses: Seventh report of the International Committee on Taxonomy of Viruses (pp 691-701) Academic Press, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gentit, P., Delbos, RP., Candresse, T. et al. Characterization of a New Nepovirus Infecting Apricot in Southeastern France: Apricot Latent Ringspot Virus. European Journal of Plant Pathology 107, 485–494 (2001). https://doi.org/10.1023/A:1011274610537
Issue Date:
DOI: https://doi.org/10.1023/A:1011274610537