Abstract
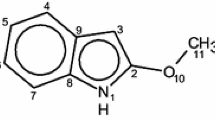
An extensive theoretical study of the stereoisomers of tetrahydrocannabinols has been performed at the ab initio HF/6-31G* and B3LYP/6-31G* levels. Effects of solvation were calculated with the Onsager model (with full geometry optimization), SCRF with Tomasi's PCM, and isodensity polarization continuum models. Single-point MP2//HF/6-31G* calculations were carried out. Frequency calculations for all the isomers at the HF/6-31G* level and for two natural isomers Δ1-THC-RR and Δ6-THC-RR at the B3LYP/6-31G* level were performed. Our results support the findings of the previous AM1 studies that the orientation of the carbocyclic ring and its C1 substituent with respect to the phenyl group hydroxyl oxygen play the major role in the activity. The calculated values of the LUMO energy (lowest unoccupied molecular orbital) and the hardness of the stereoisomers show that for the trans isomers it is easier to remove one electron from its HOMO (highest occupied molecular orbital) to the LUMO and easier to accept an electron from the receptor binding site than for the cis isomers. Combining geometric features (the orientation of the carbocyclic ring and its C1 substituent with respect to the phenyl group hydroxyl oxygen) with electronic features (LUMO and hardness), we explain the activity differences among the stereoisomers.
Similar content being viewed by others
References
Gaoni, Y. and Mechoulam, R., J. Amer. Chem. Soc., 86 (1964) 1646.
Mechoulam, R. and Gaoni, Y., Fortschr. Chem. Org. Naturst., 25 (1967) 175.
Razdan, R.K., Pharmacol. Rev., 38 (1986) 75.
Makriyannis, A. and Rapaka, R.S., Life Sci., 47 (1990) 2173.
Melvin, L.S., Milne, G.M., Johnson, M.R., Wilken, G.H. and Howlett, A.C., Drug Design Disc., 13 (1995) 155.
Johnson, M.R. and Melvin, L.S., in Mechoulam, R. (Ed.), Cannabinoids as Therapeutic Agents, CRC Press, Boca Raton, 1986, pp. 121-145.
Melvin, L.S. and Johnson, M.R., NIDA Res. Monogr., 79 (1987) 31.
Devane, W.A., Dysarz, F.A. III, Johnson, M.R., Melvin, L.S. and Howlett, A.C., Mol. Pharmacol., 34 (1988) 605
Devane, W.A. Thesis, St. Louis University, St. Louis, MO, 1989.
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. and Bonner, T.I., Nature, 346 (1990) 561.
Munro, S., Thomas, K.L. and Abu-Shaar, M., Nature, 365 (1993) 61.
Mechoulam, R. and Edery, H., in Mechoulam, R. (Ed.), Marijuana Chemistry, Metabolism, Pharmacology and Clinical Effects, Academic Press, New York, NY, 1973, pp. 101.
Jones, G., Pertwee, R.G., Gill, E.W., Paton, W.D.M., Nillsson, I.M., Widman, M. and Agurell, S., Biochem. Pharmacol., 23 (1974) 439.
Martin, B.R., Balster, R.L., Razdan, R.K., Harris, L.S. and Dewey, W.L., Life Sci., 29 (1981) 565.
Archer, R.A., Boyd, D.B., Demarco, P.V., Tyminski, I.J. and Allinger, N.L., J. Am. Chem. Soc., 92 (1970) 5200.
Tamir, I., Mechoulam, R. and Meyer, A., J. Med. Chem., 23 (1980) 220.
Reggio, P.H. and Mazurek, A.P., J. Mol. Struct. (Theochem), 149 (1987) 331.
Johnson, M.R., Melvin, L.S. and Milne G.M., Life Sci., 31 (1982) 1703.
Reggio, P.H., in Rapaka, R.S. and Makriyannis A. (Eds.), Structure Activity Relationships of the Cannabinoids, National Institute on Drug Abuse, Washington, DC, 1987, Monograph 79, pp. 15.
Reggio, P.H., Int. J. Quant. Chem., 44 (1992) 165.
Reggio, P.H., Greer, K.V. and Cox, S.M., J. Med. Chem., 32 (1989) 1630.
Reggio, P.H., Panu, A.M. and Miles, S., J. Med. Chem., 36 (1993) 1761.
Thomas, B.F., Compton, D.R., Martin, B.R. and Semus, S.F., Mol. Pharmacol., 40 (1991) 656.
Bodor, N.S. and Huang, M.-J., Int. J. Quant. Chem., 61 (1997) 127.
Sprague, J.T., Tai, J.C., Yuh, Y. and Allinger, N., J. Comput. Chem., 8 (1987) 581.
Dewar, M.J.S., Zoebisch, E.G., Healy, E.F. and Stewart, J.J.P., J. Am. Chem. Soc., 107 (1985) 3902.
Frish, M.J., Trucks, G.W., Schlegel, H.B., Gill, P.M.W., Johnson, B.G., Robb, M.A., Cheeseman, J.R., Keith, T., Petersson, G.A., Montgomery, J.A., Rahgavachari, K., Al-Laham. M.A., Zakrzewski, V.G., Ortiz, J.V., Foresman, J.B., Cioslowski, J., Stefanov, B., Nanayakkara, A., Challacombe, M., Peng, C.Y., Ayala, P.Y., Chen, W., Wong, M.W., Andres, J.L., Replogle, E.S., Goperts, R., Martin, R.L., Fox, D.J., Binkley, J.S., Defrees, D.J., Baker, J., Stwaert, J.J.P., Head-Gordon, M., Gonzalez, C. and Pople, J.A., GAUSSIAN 94, Revision E.2, Gaussian, Inc., Pittsburgh, PA, 1995.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Zakrzewski, V.G., Montgomery, J.A. Jr., Stratmann, R.E., Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O., Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S., Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Andres, J.L., Gonzalez, C., Head-Gordon, M., Replogle, E.S. and Pople, J.A., GAUSSIAN 98; Revisions A.6 and A.7, Gaussian,Inc., Pittsburgh, PA, 1998.
Onsager, L., J. Am. Chem. Soc. 58 (1936) 1486.
Miertus, S. and Tomasi, J., J. Chem. Phys. 65 (1982) 239.
Miertus, S., Swcrocco, E. and Tomasi, J., J. Chem. Phys. 55 (1981) 117.
Foresman, J.B., Keith, T.A., Wiberg, K.B., Snoonian, J. and Frisch, M.J., J. Phys. Chem. 100 (1996) 16098.
Becke, A.D., Phys. Rev. A38 (1988) 3098.
Lee, C., Yang, W. and Parr, R.G., Phys. Rev. B 37 (1988) 785.
Razdan, R.K., Pharmacol. Rev. 38 (1986) 75.
Thomas, B.F., Compton D.R. and Martin, B.R., J. Pharmacol. Exp. Ther. 255 (1990) 624.
Chiang, J.F. and Bauer, S.H., J. Am. Chem. Soc. 91 (1969) 1898.
Parr, R.F. and Pearson, R.G., J. Am. Chem. Soc. 105 (1983) 7512.
Bauschlicher, C.W. and Partridge, H., J. Chem. Phys. 103 (1995), 1788.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Huang, MJ., Leszczynski, J. An ab initio theoretical study of the stereoisomers of tetrahydrocannabinols. J Comput Aided Mol Des 15, 323–333 (2001). https://doi.org/10.1023/A:1011187218375
Issue Date:
DOI: https://doi.org/10.1023/A:1011187218375