Abstract
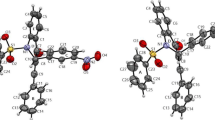
The synthesis and structural characterisation of three new macrocyclicbis-phenol A cyclophane ethers are described. The solid state structuresof two of the cyclophanes were determined by single crystal X-raydiffraction. Cyclophane 3 crystallises in the orthorhombic spacegroup Pbca with unit cell dimensions of a = 11.533(7), b = 29.383(8),c = 14.927(8) Å and cyclophane 4 in the monoclinic spacegroup P21/n with cell dimensions of a = 11.585(4), b = 11.839(2), c = 18.866(2) Å, β = 94.48(2)°.The X-ray crystalstructures reveal distorted conformations, thus supporting the weak bindingof quats in solution observed by the NMR studies. In the crystalline state bothmacrocycles were found to form self-complementary dimers held together byweak intermolecular π-π and CH-π interactions. The bindingbehaviour towards a series of tetralkylammonium cations was determinedby 1H NMR titration in CDCl3 solution. The interactions between the hosts and the quats were clearly detectable but too weak to be translated into meaningful equilibrium constants.
Similar content being viewed by others
References
J.C. Ma and D.A. Dougherty: Chem. Rev. 97, 1303 (1977) and references therein.
M. Nishio, M. Hirota and Y. Umezawa: The CH/π Interaction. Evidence, nature and consequences, (Methods in Stereochemical Analysis), Wiley-VCH, Inc. (1998).
R. Méric, J.-M. Lehn and J.-P. Vigneron: Bull. Soc. Chim. Fr. 131, 579 (1994).
D.A. Stauffer and D.A. Dougherty: Tetrahedron Lett. 29, 6039 (1988).
A. Collet, J.-P. Dutasta and B. Lozach: Bull. Soc. Chim. Belg. 99, 617 (1990).
L. Garel, B. Lozach, J.-P. Dutasta and A. Collet: J. Am. Chem. Soc. 115, 11652 (1993).
S. Pappalardo and M.F. Parisi: J. Org. Chem. 61, 8724 (1996).
A. Casnati, P. Jacopozzi, A. Pochini, F. Ugozzoli, R. Cacciapaglia and L. Mandolini: Tetrahedron 51, 591 (1995).
B. Masci, Tetrahedron 51, 5459 (1995).
B. Masci, M. Finelli and M. Varrone: Chem. Eur. J. 44, 2018 (1998).
R. Arnecke, V. Böhmer, R. Cacciapaglia, A. Dalla Cort and L. Mandolini: Tetrahedron 53, 4901 (1997).
A. Cattani, A. Dalla Cort and L. Mandolini: J. Org. Chem. 60, 8313 (1995).
S. Roelens and R. Torriti: J. Am. Chem. Soc. 120, 12443 (1998).
W.J. Bailey and E. Fujiwara: J. Am. Chem. Soc. 77, 165 (1955).
G.M. Sheldrick: Acta Crystallogr. A46, 467 (1990).
G.M. Sheldrick: SHELXL-97-A Program for Crystal Structure Refinement 1997, University of Göttingen, Germany.
J.B. Armitage and M.C. Whiting: J. Chem. Soc. 2005 (1952).
J. Ratilainen, K. Airola, M. Nieger, M. Böhme, J. Huuskonen and K. Rissanen: Chem. Eur. J. 3, 749 (1997).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nissinen, M., Dalla Cort, A., Amabile, S. et al. Bis-phenol A Cyclophanes: Synthesis, Crystal Structures and Binding Studies. Journal of Inclusion Phenomena 39, 229–234 (2001). https://doi.org/10.1023/A:1011174213426
Issue Date:
DOI: https://doi.org/10.1023/A:1011174213426