Abstract
Purpose. To develop once-a-day oral dosing regimen that provides the blood levels of cyclosporin A (CsA) in the therapeutic ranges over 24 hours.
Methods. CsA premicroemulsion concentrates (preME) were formulated from phase diagrams. Enteric-coated solid-state premicroemulsion concentrates (sME) were prepared by coating preME with enteric-coating matrials and solidifying them. CsA was measured using high-performance liquid chromatography or radioimmunoassay.
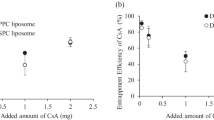
Results. PreME consisted of CsA, oil, and mixture of surfactants and a cosurfactant. PreME spontaneously formed microemulsions in aqueous medium and showed oral absorption profiles similar to Sandimmune Neoral® in dogs. Dispersion of sME in aqueous medium also formed microemulsions. Release rates of CsA from sME depended on pH and the type of enteric-coating materials and highly correlated with the extent of oral absorption. The co-administration of preME and sME (200 mg CsA) showed the maximum blood level of CsA not significantly different from that of preME (100 mg CsA) and the concentration of CsA close to the minimum therapeutic level at 24 hours.
Conclusions. The combined treatment of preME and sME provided controlled oral absorption of CsA over a 24-hour period. Such once-a-day dosing regimens will lead to increased patient compliance and reduced episodes of organ rejection after transplantation.
Similar content being viewed by others
REFERENCES
J. P. Scott and T. W. Higenbottam. Adverse reactions and interactions of cyclosporin. Med. Toxicol. Adverse Drug Exp. 3:107-127 (1988).
H. Zachariae. Renal toxicity of long-term cyclosporin. Scand. J. Rheumatol. 28:65-68 (1999).
J. M. Gijtenbeek, M. J. van den Bent, and C. J. Vecht. Cyclosporine neurotoxicity: A review. J. Neurol. 246:339-346 (1999).
B. J. Nankivell, M. Hibbins, and J. R. Chapman. Diagnostic utility of whole blood cyclosporine measurements in renal transplantation using triple therapy Transplantation 58:989-996 (1994).
R. M. Ferguson and R. Fidelus-Gort. The in vitro assessment of the immunosuppressive effect of fractionated total lymphoid irradiation in renal allotransplantation. Transplant Proc. 14:2350-2356 (1983).
P. P. Constantinides. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm. Res. 12:1561-1572 (1995).
J. M. Kovarik, E. A. Mueller, J. B. Van Bree, W. Tetzloff, and K. Kutz. Reduced inter-and intraindividual variability in cyclosporine pharmacokinetics from a microemulsion formulation. J. Pharm. Sci. 83:444-446 (1994).
C. K. Kim, E. J. Lee, M. K. Lee, J. K. Park, H. J. Shin, H. G. Choi, S. W. Lee, S. J. Lim, Z. G. Gao, and I. S. Kim. Bioequivalence of cyclosporin A hard capsules. J. Applied Pharmacol. 6:296-302 (1998).
Z. G. Gao, H. G. Choi, H. J. Shin, K. M. Park, S. J. Lim, K. J. Hwang, and C. K. Kim. Physicochemical characterization and evaluation of a microemulsion system for oral delivery of cyclosporin A. Int. J. Pharm. 161:75-86 (1998).
P. Keown, D. Landsberg, P. Halloran, A. Shoker, D. Rush, J. Jeffery, D. Russell, C. Stiller, N. Muirhead, E. Cole, L. Paul, J. Zaltzman, R. Loertscher, P. Daloze, R. Dandavino, A. Boucher, P. Handa, J. Lawen, P. Belitsky, and P. Parfrey. A randomized, prospective multicenter pharmacoepidemiologic study of cyclosporine microemulsion in stable renal graft recipients. Report of the Canadian neoral renal transplantation study group. Transplantation 62:1744-1752 (1996).
B. D. Kahan and J. Grevel. Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation 46:631-644 (1988).
S. L. Myers and M. L. Shively. Preparation and characterization of emulsifiable glasses: Oil-in-water and water-in-oil-in-water emulsions. J. Colloid Interface Sci. 149:271-278 (1992).
C. K. Kim, S. A. Ryuu, K. M. Park, S. J. Lim, and S. J. Hwang. Preparation and physicochemical characterization of phase inverted water/oil microemulsion containing cyclosporin A. Int. J. Pharm. 147:131-134 (1997).
E. S. Swenson and W. J. Curatolo. Intestinal permeability enhancement for proteins, peptides and other polar drugs—Mechanisms and potential toxicity. 2. Adv. Drug. Deliv. Rev. 8:39-92 (1992).
M. M. Nerurkar, P. S. Burton, and R. T. Borchardt. The use of surfactants to enhance the permeability of peptides through Caco-2 cells by inhibition of an apically polarized efflux system. Pharm. Res. 13:528-534 (1996).
K. M. Park and C. K. Kim. Preparation and evaluation of flurbiprofen-loaded microemulsion for parental delivery. Int. J. Pharm. 181:173-179 (1999).
K. M. Park, M. K. Lee, K. J. Hwang, and C. K. Kim, Phospholipid-based microemulsions of flurbiprofen by the spontaneous emulsification process. Int. J. Pharm. 183:145-154 (1999).
J. Drewe, C. Beglinger, and T. Kissel, The absorption site of cyclosporin in the human gastrointestinal tract. Br. J. Clin. Pharmacol. 33:39-43 (1992).
D. Attwood. Microemulsions. In J. Kreuter (ed.), Colloidal Drug Delivery Systems, Marcel Dekker Inc., New York, 1994 pp. 31-65.
P. Keown and D. Niese, Cyclosporine microemulsion increases drug exposure and reduces acute rejection without incremental toxicity in de novo renal transplantation. InternationalSandimmun Neoral Study Group. Kidney Int. 54:938-944 (1998).
A. Hoffman, H. D. Danenberg, I. Katzhendler, R. Shuval, D. Gilhar, and M. Friedman. Pharmacodynamic and pharmacokinetic rationales for the development of an oral controlled-release amoxicillin dosage form. J. Control. Release 54:29-37 (1998).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, CK., Shin, HJ., Yang, SG. et al. Once-a-Day Oral Dosing Regimen of Cyclosporin A: Combined Therapy of Cyclosporin A Premicroemulsion Concentrates and Enteric Coated Solid-State Premicroemulsion Concentrates. Pharm Res 18, 454–459 (2001). https://doi.org/10.1023/A:1011046109078
Issue Date:
DOI: https://doi.org/10.1023/A:1011046109078