Abstract
Purpose. To quantitatively describe the pharmacokinetics of valproic acid (VPA) in guinea pig serum (total [Cf+b] and free [Cf]), cerebrospinal fluid (CSF) [C]CSF and tears [C]T using a simple kinetic model, and to examine whether [Cf] and [C]CSF can be predicted by [C]T using the resulting pharmacokinetic parameters.
Methods. [Cf+b], [Cf], [C]CSF and [C]T were determined after bolus i.v. injection of 10 or 20 mg/kg VPA using GC/ECNCI/MS.
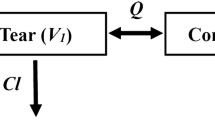
Results. [Cf+b] could be quantitatively described by a two compartment model with linear elimination kinetics. [Cf] was separately analyzed using multi-exponential equations. [C]CSF was analyzed using a simple kinetic model in which the CSF compartment is independently connected with the serum compartment by the apparent diffusion constants (K INCSF and K OUTCSF). [C]T was analyzed using the same simple kinetic model used for [C]CSF. The values of [C]CSF and [Cf] in the steady state can be represented by the following equations; [C]CSF = K INCSF/K OUTCSF × [Cf], [Cf] = K OUTT/K INT × [C]T, and indicating that [Cf] and [C]CSF can be predicted by [C]T using the resulting pharmacokinetic parameters.
Conclusions. The measurement of [C]T which can be collected non-invasively and estimated the pharmacokinetic parameters for [Cf], [C]CSF, and [C]T might be a very useful method for TDM of VPA.
Similar content being viewed by others
REFERENCES
R. L. O. Semmes and D. D. Shen. Nonlinear binding of valproic acid (VPA) and E-Δ2-valproic aid to rat plasma proteins. Pharm. Res. 7:461-467 (1990).
P. L. Morselli. Development of physiological variables important for drug kinetics. In P. L. Morselli, C. E. Pippenger, J. K. Penry (eds.), Antiepileptic Drug Therapy in Pediatrics, Raven Press, New York 1983 pp. 1-12.
R. Gugler and G. Mueller. Plasma protein binding of valproic acid in healthy subjects and in patients with renal disease. Br. J. Clin. Pharmacol. 5:441-446 (1978).
M. Nakajima, S. Yamato, K. Shimada, S. Sato, S. Kitagawa, A. Honda, J. Miyamoto, J. Shoda, M. Ohya, and H. Miyazaki. Assessment of tear concentrations on therapeutic drug monitoring. I. Determination of valproic acid in tears by gas chromatography/mass spectrometry with EC/NCI mode. Ther. Drug. Monit. 22:716-722 (2000).
F. Monaco, S. Piredda, R. Mutani, C. Mastropaolo, and M. Tondi. The free fraction of valproic acid in tears, saliva, and cerebrospinal fluid. Epilepsia 23:23-26 (1982).
W. Loscher and H.-H. Frey. Kinetics of penetration of common antiepileptic drugs into cerebrospinal fluid. Epilepsia 25:346-352 (1984).
R. D. Schoenwald, Y-S Yang, E. Xia, and C. F. Barfknecht. Uptake of N,N-dimethyl-2-phenylethylene HCl into acini cells removed from rabbit lacrimal glands. J. Ocular Pharmacol. Ther. 14:253-262 (1998).
Y. Horibe, K. Hosoya, K-J. Kim, and V. H. L. Lee. Carrier-mediated transport of monocarboxylate drugs in the pigmented rabbit conjunctiva. Invest. Ophthalmol. Vis. Sci. 39:1436-1443 (1998).
S. Sato and A. Koshiro. Pharmacokinetic analysis of chlorpromazine in rat serum, cerebrospinal fluid and striatum. Biol. Pharm. Bull. 18:593-599 (1995).
M. Berman, E. Shahn, and M. F. Weiss. The routine fitting of kinetic data to models: A mathematical formalism for digital computers. Biophys. J. 2:275-287 (1962).
J. H. Zar. Biostatistical Analysis 2nd Ed, Prentice-Hall Inc., New Jersey, 1984.
H-Y. Yu and Y-Z. Shen. Dose-dependent inhibition in plasma protein binding of valproic acid during continued treatment in guinea pigs. J. Pharm. Pharmacol. 44:408-412 (1992).
S. Sato and A. Koshiro. Protein binding of chlorpromazine in vivo and in vitro: Effect of chlorpromazine metabolite on chlorpromazine protein binding in rat. Biol. Pharm. Bull. 18:586-892 (1995).
H. Sato, E. Okezaki, S. Yamamoto, O. Nagata, H. Kato, and A. Tsuji. Entry of the new quinolone antibacterial agents of ofloxacin and NY-198 into the central nervous system in rats. J. Pharmacobio-Dyn. 11:386-394 (1988).
J. M. Collins and R. L. Dedrick. Distributed model for drug delivery to CSF and brain tissue. Am. J. Physiol. 245:R303-R310 (1983).
P. L. Golden, K. R. Brouwer, and G. M. Pollack. Assessment of valproic acid serum-cerebrospinal fluid transport by microdialysis. Pharm. Res. 10:1765-1771 (1993).
G. M. Pollack and D. D. Shen. A timed intravenous pentylenetetrazol infusion seizure model for quantitating the anticonvulsant effect of valproic acid in the rat. J. Pharmacol. Meth. 13:135-146 (1985).
H. H. Frey and W. Loscher. Distribution of valproate across the interface between blood and cerebrospinal fluid. Pharmacology 17:637-642 (1978).
A. Lucke, T. Mayer, U. Altrup, A. Lehmenkuhler, R. Dusing, and E.-J. Speckmann. Simultaneous and continuous measurement of free concentration of valproate in blood and extracellular space of rat cerebral cortex. Epilepsia 35:922-926 (1994).
D. D. Shen, G. A. Ojemann, R. L. Rapport, R. L. Dills, P. N. Friel, and R. H. Levy. Low and variable presence of valproic acid in human brain. Neurology 42:582-585 (1992).
E. M. Conford, C. P. Diep, and W. M. Pardridge. Blood-brain barrier transport of valproic acid. J. Neurochem. 44:1541-1550 (1985).
S. S. Chrai, T. F. Patton, A. Mehta, and J. R. Robinson. Lacrimal and instilled fluid dynamics in rabbit eyes. J. Pharm. Sci. 62:1112-1121 (1973).
J. C. Keister, E. R. Cooper, P. J. Missel, J. C. Lang, and D. F. Hager. Limits on optimizing ocular drug delivery. J. Pharm. Sci. 80:50-53 (1991).
A. Shimizu, N. Yokoi, K. Nishida, S. Kinoshita, and K. Akiyama. Fluorophotometric measurement of tear volume and tear turnover rate in human eyes. J. Jpn. Ophthalmol. Soc. 97:1047-1052 (1993).
N. J. van Haeringen. Secretion of drugs in tears. Curr. Eye. Res. 4:485-488 (1985).
V. Baeyens and R. Gurny. Chemical and physical parameters of tears relevant for the design of ocular drug delivery formulations. Pharmaceutica Acta Helvetiae. 72:191-202 (1997).
A. K. Mircheff. Lacrimal fluid and electrolyte secretion: A review. Curr. Eye. Res. 8:607-617 (1989).
D. A. Dartt, M. Moller, and J. H. Poulsen. Lacrimal gland electrolyte and water secretion in the rabbit: localization and role of (Na+ + K+)-activate ATPase. J. Physiol. Lond. 321:557-569 (1981).
A. K. Mitra and T. J. Mikkelson. Mechanism of transcorneal permeation of pilocarpine. J. Pharm. Sci. 77:771-775 (1988).
S. W. Friedrich, Y-L Cheng, and B. A. Saville. Theoretical corneal permeation model for ionizable drugs. J. Ocul. Pharmacol. 9:229-249 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sato, S., Kitagawa, S., Nakajima, M. et al. Assessment of Tear Concentrations on Therapeutic Drug Monitoring. II. Pharmacokinetic Analysis of Valproic Acid in Guinea Pig Serum, Cerebrospinal Fluid, and Tears. Pharm Res 18, 500–509 (2001). https://doi.org/10.1023/A:1011010528642
Issue Date:
DOI: https://doi.org/10.1023/A:1011010528642