Abstract
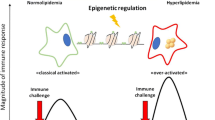
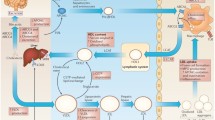
Current investigation on the origin of atherosclerosis has initiated an intense debate over whether atherosclerosis results from hypercholesterolemia or an inappropriate immune response to vascular injury. Although the role of the immune system has been questioned, the overwhelming body of evidence clearly indicates that atherogenesis is initiated by the interplay between cholesterol and cellular secretion of cytokines (especially IL-6) and apolipoprotein ‘E’ within the arterial wall. Recent studies have revealed that cells possess two cholesterol-sensors: (a) Receptor-Ck which senses the extracellular cholesterol and initiates signalling pathway responsible for the regulation of genes involved in the cell cycle, cell death, cellular cholesterol homeostasis and cytokines including IL-6; (b) LxRα which senses intracellular oxysterols and controls genes involved in cell death, cellular cholesterol homeostasis and cytokine IL-8. These cholesterol sensors define the molecular mechanism responsible for cholesterol-depended regulation of cellular synthesis and secretion of cytokines (IL-6, IL-8) within arterial wall. On the basis of this mechanism, presence of cholesterol and its oxy-derivative in the modified LDL will result in transient activation/deactivation of Receptor-Ck-dependent genes which will give rise to repeated cycles of growth coupled with apoptosis leading to a situation where apoptotic-deficient cells in the arterial wall, would be selected resulting in their accumulation and formation of oligoclonal atherosclerotic plaque.
Similar content being viewed by others
References
Wick G, Schelt G, Amberger A, Kleindienst R, Xu Q: Is atherosclerosis an immunologically mediated disease? Immunol Today 16: 27–33, 1995
Fyte AI, Qiao JH, Lusis AJ: Immunodeficient mice develop typical atherosclerotic fatty streak when fed an atherogenic diet. J Clin Invest 94: 2516–2520, 1994
Laman JD, deSmet BJGL, Schoneveld A, Meurs MV: CD4o-CD40L interaction in atherosclerosis. Immunol Today 18: 272–277, 1997
Mehta U, Kaul D: Nature of aortic smooth muscle cellular activity induced by cholesterol incorporation through an LDL-receptor independent pathway: Role of trifluoperazine on such activity. Exp Mol Pathol 55: 13–24, 1991
Lusis AJ, Navab M: Lipoprotein oxidation and gene expression in arterial wall. New opportunities of pharmacological intervention in atherosclerosis. Biochem Pharmacol 46: 2119–2136, 1993
Palinski W, Rozenfeld ME, Vla-HerHuala S, Gurtner GC, Socker SS, Butler SW et al.: Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA 86: 1372–1376, 1989
Keys A: Cornary heart disease - the global picture. Atherosclerosis 22: 149–192, 1975
Buchwald H: Cholesterol inhibition, cancer and chemotherapy. Lancet 339: 1154–1156, 1992
Vitols S, Norgen S, Juliusson G, Tabtidies L, Luthman H: Multilevel regulation of low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenyme A reductase gene expression in normal and leukemic cells. Blood 84: 2689–2698, 1994
Brown MS, Goldstein SL: A receptor mediated pathway for cholesterol homeostasis. Science 232: 37–47, 1986
Goldstein JL, Brown MS: Regulation of the mevalonate pathway. Nature 343: 425–430, 1990
Vaux DL, Strasser A: The molecular biology of apoptosis. Proc Natl Acad Sci USA 93: 2239–2244, 1996
Roma P, Catapano AL, Bertulli SM, Varesi L, Fumagelli R, Bernini F: Oxidized LDL increases free cholesterol and fail to stimulate cholesterol esterfication in murine macrophages. Biochem Biophys Res Commun 171: 123–131, 1990
Young SG, Parthasarathy S: Why are low-density lipoproteins atherogenic? West J Med 160: 153–164, 1994
Ferman BM, Ruan B, Chen J, Schroepter GJ Jr, Evans RM: The orphan nuclear receptor LxRα is positively and negatively regulated by distinct products of mevalonate metabolism. Proc Natl Acad Sci USA 94: 10588–10593, 1997
Ntambi JM: Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J Lipid Res 40: 1549–1558, 1999
Kaul D: Do human platelets posses LDL-receptor specific for apoprotein ‘B’ or cholesterol? Curr Sci 65: 883–886, 1993
Kaul D: Receptor Ck and leukemogenesis. Leuk Res 22: 389–394, 1998
Goel R, Kaul D, Varma S: Lymphocytes from CML patients lack 47 kDa factor having affinity for genomic sterol regulatory sequence. Leuk Res 20: 877–879, 1996
Goel R, Varma S, Kaul D: Sterol dependent LDL-receptor gene transcription from normal and CML patients gene transcription from normal and CML patients. Cancer Lett 107: 193–198, 1996
Goel R, Singh J, Kaul D: Receptor-Ck dependent signalling regulates LDL receptor transcription. Mol Cell Biochem 169: 79–83, 1997
Sujimoto Y, Croce CM: Analysis of the structure transcripts and protein products of Bcl-2, the gene involved in follicular lymphoma. Proc Natl Acad Sci USA 83: 5214–5218, 1986
Herber B, Truss M, Beato M, Muller R: Inducible regulating elements in the human cyclin ‘D’ promoter. Oncogene 9: 1295–1304, 1994
Shimano H, Horton JD, Shimomura L, Hammer RE, Brown MB, Godstein JL: Isoform Ic of sterol regulatory element binding protein in less active than isoform Ia in livers of transgenic mice in cultured cells. J Clin Invest 99: 846–854, 1997
Moolenaar WA, Kruijer W, Tilly BC, Verlann I, Bierman AJ, deLaat SW: Growth factor-like action of phosphatidic acid. Nature 323: 171–173, 1986
Kaur M, Kaul D, Sobti RC: Receptor-Ck dependent regulation of genes involved in the cell cycle. Mol Cell Biochem 181: 137–142, 1998
Kaul D, Kaur M: Receptor-Ck regulates the expression of cyclin-dependent kinase inhibitors. Mol Cell Biochem 200: 183–186, 1999
Kaul D, Kaur M: Receptor-Ck central cholesterogenesis and DNA replication in HepG2 cells. Curr Sci 77: 592–594, 1999
Brown MS, Goldstein JL: The SREBP Pathway-regulation of cholesterol metabolism by proteolysis of a membrane bond transcription factor. Cell 89: 331–340, 1997
Stark AC, Weinberger O, Weinberger J: Common double and single stranded DNA binding factor for a sterol regulating element. Proc Natl Acad Sci USA 89: 2180–2184, 1992
Lopez D, Ness GC: Inhibitors of 3-hydrocy-3-methylglutaryl coenzyme. A reductase unmask transcriptional regulation of hepatic low density lipoprotein gene expression by dietary cholesterol. Arch Biochem Biophys 344: 215–219, 1999
Linette GP, Li Y, Roth K, Korsmeyer SJ: Cross-talk between cell death and cell cycle progression: Bcl-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA 93: 9545–9552, 1996
Fielding CJ, Fielding PE: Molecular physiology of reverse cholesterol transport. J Lipid Res 36: 211–228, 1995
Hill SA, McQueen MJ: Reverse cholesterol transport - a review of the process and its clinical implications. Clin Biochem 30: 517–525, 1997
Barroaus A, Jaspard B, Barbaras R, Chap H, Perret B, Collect X: Prebeta HDL: Structure and metabolism. Biochem Biophys Acta 1300: 73–85, 1996
Yamashita S, Maruyama T, Ken-ichi H, Sakai N, Nakajima N, Matsuzana Y: Molecular mechanisms, lipoprotein abnormalities and atherogenecity of hyperalphalipoproteinemia. Atherosclerosis 152: 271–285, 2000
Rubin EM, Krauss RM, Spangler EA, Uerstuyft JG, Cliff SM: Inhibition of early atherogenesis in transgenic mice by human apolipoprotein A-I. Nature 353: 265–267, 1991
Piero C, Carmen G, Jose MO, Jose CR, Javier L: Induction of apolipoprotein ‘E’ gene expression in human and experimental atherosclerotic lesions. Biochem Biophys Res Commun 168: 733–740, 1990
Mahley RW, Innerarity TL: Lipoprotein receptors and cholesterol homeostasis. Biochem Biophys Acta 737: 197–222, 1983
Laskowitz DT, Lee DM, Schemechel D, Staats HF: Altered immune responses in apolipoprotein ‘E'-deficient mice. J Lipid Res 41: 613–620, 2000
Cader AA, Steinberg FM, Mazzone T, Chait A: Mechanisms of enhanced macrophage apo-'E’ secreted by oxidized LDL. J Lipid Res 38: 981–991, 1997
Schroepfer GJ Jr: Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol Rev 80: 361–554, 2000
Mehta U, Kaul D: Nature of aortic smooth muscle cellular activity induced by cholesterol-incorporation through LDL-receptor independent pathway: Preventive role of Trifiperazine on such activity. Exp Mol Pathol 55: 13–24, 1991
Liu Y, Hulten LM, Wiklund O: Macrophages isolated from human atherosclerotic plaques produce IL-8 and oxysterols may have a regulatory function for IL-8 production. Arterioscler Thromb Vasc Biol 17: 317–323, 1997
Rus HG, Vlaicu R, Niculescu F: Interleukin-6 and interleukin-8 protein and gene expression in human arterial atherosclerotic wall. Atherosclerosis 127: 263–271, 1996
Seino Y, Ikeda U, Ikeda M, Yamamoto K, Misawa Y, Hasegawa T, Kano S, Shimado K: Interleukin-6 gene transcripts are expressed in human atherosclerotic lesions. Cytokines 6: 87–91, 1994
Ikeda U, Ikeda M, Seino Y, Takashashi M, Kano S, Shinada K: Interleukin-6 gene transcripts are expressed in atherosclerotic lesions of genetically hyperlipidemic rabbits. Atherosclerosis 92: 213–218, 1992
Yudkin JS, Kumari M, Humphries SE, Mohamad-Ali V: Inflammation. Obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 148: 209–214, 2000
Skretting G, Gjernes E, Prydz H: Regulation of lecithin: Cholesterol acyltransferase by TGF-beta and interleukin-6. Biochem Biophys Acta 1255: 267–272, 1995
Zuckerman SH, Evans GF, O'Neal A: Cytokine regulation of macrophage apo-'E’ secretion: Opposing effects of GM-CSF and TGF-beta. Atherosclerosis 96: 203–214, 1992
Mech F, Schonbeck U, Sukhova GK, Atkinson E, Libby P: Reduction of atherosclerosis in mice by inhibiting CD4o signalling. Nature 394: 200–203, 1998
Dechanet J, Grosset C, Taupin JL, Merville P, Bauchereau J, Ripoche J, Moreau JF: CD4o ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol 159: 5640–5647, 1997
Urashima M, Chauhan D, Hatziyanni M, Ogata A, Hollenbaugh D, Aruffo A, Anderson KC: CD4o ligand triggers interleukin-6 mediated B-cell differentiation. Leuk Res 20: 507–515, 1996
Clark EA, Shu G: Association between IL-6 and CD4o signalling. IL-6 induced phosphorylation of CD4o receptors. J Immunol 145: 1400–1406, 1990
Sukovich DA, Kauser K, Shirley FD, Delvecchio V, Halks-Miller M, Rubanyi GM: Expression of interleukin-6 in atherosclerotic lesions of male apo ‘E'-knockout mice: Inhibition by 17 β-estradiol. Arterioscler Thromb Vasc Biol 18: 1498–1505, 1998
Moolenaar WH, Kruijer W, Tilly BC, Verlaan I, Biermanp AJ, de Latt SW: Growth factor-like action of phosphatidic acid. Nature 323: 171–173, 1986
Freeman EJ: The Ang II-induced growth of vascular smooth muscle cells involves a phospholipase ‘D'-mediated signalling mechanism. Arch Biochem Biophys 374: 363–370, 2000
Luo Y, Tall AR: Sterol upregulation of human CETP expression in vitro and in transgenic mice by an LXR element. J Clin Invest 105: 513–520, 2000
Leszczynski D, Zhao Y, Luokkamaki M, Foegh ML: Apoptosis of smooth muscle cells: Protein kinase ‘C’ and oncoprotein Bcl-2 are involved in regulation of apoptosis in nontransformed Rat vascular smooth muscle cells. Am J Pathol 145: 1265–1270, 1999
Jiang C, Ting AT, Seed B: PPAR-g agonists inhibit production of monocyte inflammatory cytokines. Nature 391: 82–88, 1998
Berg K: Twin research in coronary heart disease. Prog Clin Biol Res 69C: 117–130, 1981
Brada M: Bcl-2 gene: Current relevance to clinical oncology. Eur J Cancer 28: 270–272, 1992
Bissonette RP, Echeverri F, Maliboubi A, Green DR: Apoptotic cell death induced by c-myc is inhibited by Bcl-2. Nature 359: 552–554, 1992
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kaul, D. Molecular link between cholesterol, cytokines and atherosclerosis. Mol Cell Biochem 219, 65–71 (2001). https://doi.org/10.1023/A:1011006707414
Issue Date:
DOI: https://doi.org/10.1023/A:1011006707414