Abstract
Purpose. To examine the potential of raffinose as an excipient in stabilizing protein and to study the effect of sucrose/raffinose mass ratios on the stability of co-lyophilized protein and amorphous solids during storage at an elevated temperature.
Methods. Glucose-6-phosphate dehydrogenase (G6PDH) was co-lyophilized with sucrose and raffinose mixed at different mass ratios. The activity of dried G6PDH was monitored during storage at 44°C. Thermal properties of sucrose/raffinose matrices were determined by differential scanning calorimetry (DSC).
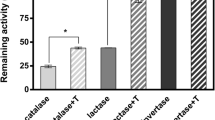
Results. Mass ratios of sucrose to raffinose did not affect the recovery of G6PDH activity after freeze-drying, but significantly affected the stability of freeze-dried G6PDH during storage. The sucrose-alone formulation offered the best enzyme stabilization during storage. With increasing fraction of raffinose, the G6PDH stability decreased, sugar crystallization inhibited, and crystal-melting temperature increased.
Conclusions. Despite the higher Tg of the formulations with higher fraction of raffinose, they provided less protection for G6PDH than did sucrose alone during storage. Our data do not support the prediction from recent thermophysical studies that raffinose should be superior to sucrose and trehalose as a potential excipient or stabilizer.
Similar content being viewed by others
REFERENCES
T. Arakawa, S. J. Prestrelski, W. C. Kenney, and J. F. Carpenter. Factors affecting short-term and long-term stability of proteins. Adv. Drug Deliv. Rev. 10:1-28 (1993).
J. F. Carpenter and J. H. Crowe. An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry 28:3916-3922 (1989).
F. Franks. Long-term stabilization of biologicals. Bio/Technology 12:253-256 (1994).
F. Franks. Thermochemical properties of amorphous saccharides: their role in enhancing pharmaceutical product stability. Biotech. Genet. Eng. Rev. 16:281-292 (1999).
B. J. Aldous, A. D. Auffret, and F. Franks. The crystallisation of hydrates from amorphous carbohydrates. Cryo-Letters 16:181-186 (1995).
A. Saleki-Gerhardt, J. G. Stowell, S. R. Byrn, and G. Zografi. Hydration and dehydration of crystalline and amorphous forms of raffinose. J. Pharm. Sci. 84:318-323 (1995).
K. Kajiwara and F. Franks. Crystalline and amorphous phases in the binary system water-raffinose. J. Chem. Soc. Faraday Trans. 93:1779-1783 (1997).
K. Kajiwara, F. Franks, P. Echlin, and A. L. Greer. Structural and dynamic properties of crystalline and amorphous phases in raffinose-water mixtures. Pharm. Res. 16:1441-1448 (1999).
T. Moreira, J. Gutierrez, R. Pomes, J. Duque, and F. Franks. Effect of sucrose and raffinose on physical state and on lactate dehydrogenase activities of freeze-dried formulations. Cryo-Letters 19:115-122 (1998).
W. Q. Sun and A. C. Leopold. Cytoplasmic vitrification and survival of anhydrobiotic organisms. Comp. Biochem. Physiol. 117A:327-333 (1997).
W. Q. Sun. Function of the glassy state in seed storage stability. In A. G. Taylor and X-L. Huang (eds.), Progress in Seed Research, Communication Service of New York State Agricultural Experimental Station, New York, 1998 pp. 169-179.
S. H. Gaffney, E. Haslam, T. H. Lilley, and T. R. Ward. Homotactic and heterotactic interactions in aqueous-solutions containing some saccharides—Experimental results and an empirical relationship between saccharide solvation and solute-solute interactions. J. Chem. Soc. Faraday Trans. 84:2545-2552 (1988).
S. J. Prestrelski, N. Tedeschi, T. Arakawa, and J. F. Carpenter. Dehydration-induced conformational transitions in protein and their inhibition by stabilizers. Biophys. J. 65:661-671 (1993).
J. H. Crowe, S. B. Leslie, and L. M. Crowe. Is vitrification sufficient to preserve liposomes during freeze-drying? Cryobiology 31:355-366 (1994).
S. B. Leslie, E. Israeli, B. Lighthart, J. H. Crowe, and L. M. Crowe. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 61:3592-3597 (1995).
Y. H. Roos. Frozen state transitions in relation to freeze drying. J. Therm. Anal. 48:535-544 (1997).
L. Slade and H. Levine. Beyond water activity: Recent advances based on an alternative approach to the assessment of food quality and safety. Crit. Rev. Food. Sci. Nutr. 30:115-360 (1991).
R. J. Bellows and C. J. King. Product collapse during freeze-drying of liquid foods. AICHE Symposium Series 69 132:33-41 (1973).
F. Franks, R. H. M. Hatley, and S. F. Mathias. Materials science and the production of shelf-stable biologicals. BioPharm 4:38-42 (1991).
W. Q. Sun, A. C. Leopold, L. M. Crowe, and J. H. Crowe. Stability of dry liposomes in sugar glasses. Biophys. J. 70:1769-1776 (1996).
S. Rossi, M. P. Buera, S. Moreno, and J. Chirife. Stabilization of the restriction enzyme EcoRI dried with trehalose and other selected glass-forming solutes. Biotechnol. Prog. 13:609-616 (1997).
W. F. Wolkers, H. Oldenhof, M. Alberda, and F. A. Hoekstra. A fourier transform infrared microspectroscopy study of sugar glasses: Application to anhydrobiotic higher plant cells. Biochim. Biophys. Acta 1379:83-96 (1998).
R. H. M. Hatley. Glass fragility and the stability of pharmaceutical preparations—Excipient selection. Pharm. Dev. Technol. 2:257-284 (1997).
L. M. Crowe, D. S. Reid, and J. H. Crowe. Is trehalose special for preserving dry biomaterials? Biophys. J. 71:2087-2093 (1996).
W. Q. Sun and P. Davidson. Protein inactivation in amorphous sucrose and trehalose matrices: Effects of phase separation and crystallization. Biochim. Biophys. Acta 1425:235-244 (1998).
M. J. Pikal and D. R. Rigsbee. The stability of insulin in crystalline and amorphous solids: Observation of greater stability for the amorphous form. Pharm. Res. 14:1379-1387 (1997).
F. P. Schwarz. Enthalpy of solution of carbohydrates using a modified differential scanning calorimeter. J. Solution Chem. 25:471-484 (1996).
H. A. Iglesias, C. Schebor, M. P. Buera, and J. Chirife. Sorption isotherm and calorimetric behavior of amorphous/crystalline raffinose-water systems. J. Food Sci. 65:646-650 (2000).
T. Suzuki, K. Imamura, K. Yamamoto, T. Satoh, and M. Okazaki. Thermal stabilization of freeze-dried enzymes by sugars. J. Chem. Eng. Japan 30:609-613 (1997).
The Merck Index on CD-ROM (version 12:1), Merck and Co., Whitehouse Station, NJ, 1996.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Davidson, P., Sun, W.Q. Effect of Sucrose/Raffinose Mass Ratios on the Stability of Co-Lyophilized Protein During Storage Above the Tg. Pharm Res 18, 474–479 (2001). https://doi.org/10.1023/A:1011002326825
Issue Date:
DOI: https://doi.org/10.1023/A:1011002326825