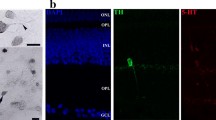
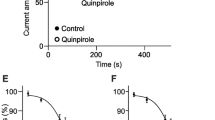
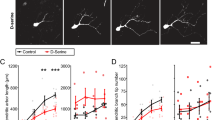
Abstract
The presence of serotonin 5-HT1A receptors and their physiological role were further characterized in the goldfish retina. The effects of the 5-HT6/7 receptor antagonists pimozide, fluphenazine and amoxapine, the 5-HT1A receptor antagonist WAY-100,135, and the alkylating agent N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline, on the 5-HT1A receptor agonist [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding to retinal membranes, were evaluated. In addition, the effects of serotonin, 8-hydroxy-2-(di-n-propylamino)tetralin, WAY-100,135, the adenylate cyclase inhibitors SQ22536 and MDL12330A, and the cyclic AMP analog 8-bromoadenosine-3′:5′ cyclic monophosphate were also studied on neuritic outgrowth from retinal explants. WAY-100,135 but not 5-HT6/7receptor antagonists inhibited [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding to retinal membranes N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline decreased [3H]8-hydroxy-2-(di-n-propylamino)tetralin binding sites up to 70%, while receptor turnover was similar to that reported in other tissues. Serotonin and 8-hydroxy-2-(di-n-propylamino)tetralin stimulated cyclic AMP production, both ex vivo and in vitro, and these increases were related to inhibition of neuritic outgrowth. The inhibitory effect was reduced by SQ22536 and by WAY-100,135, and was mimicked by 8-bromoadenosine-3′:5′cyclic monophosphate. This study supports previous findings about the role of serotonin as a regulator of axonal outgrowth during in vitro regeneration of the goldfish retina and demonstrates that this effect is mediated, at least in part, by 5-HT1A receptors through a mechanism which involves an increase of cyclic AMP levels.
Similar content being viewed by others
REFERENCES
Khawaja, X. 1995. Quantitative autoradiographic characterisation of the binding of [3H]WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res. 673:217–225.
Verge, D., Daval, G., Marcinkiewics, A., Patey, S., El, Mestikawy, S., Gozlan, H., and Hamon, M. 1986. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J. Neurosci. 6: 3474–3482.
Gozlan, H., El, Mestikawy, S., Pichat, L., Glowinski, J., and Hamon, M. 1983. Identification of presynaptic 5-HT autoreceptors using a new ligand: [3H]PAT. Nature 305:140.
Hall, M., El, Mestikawy, S., Emerit, M., Pichat, L., Hamon, M., and Gozlan, H. 1985. [3H]8-hydroxy-2-(Di-n-Propylamino) tetralin binding to pre-and postsynaptic 5-hydroxytryptamine sites in various regions of the rat brain. J. Neurochem. 44: 1685–1695.
Lima, L., Schmeer, C., and Urbina, M. 1994. 8-[3H]hydroxy-2-(Di-n-propylamino)tetralin binding sites in goldfish retina. Neurochem. Res. 19:249–255.
Plassat, J., Amlaiky, N., and Hen, R. 1993. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 44:229–236.
Chidlow, G. and Osborne, N. 1997. Antagonism of muscarinic receptors in the rabbit iris-ciliary body by 8-OH-DPAT and other 5-HT1A receptor agonists. J. Neural Transm. 104:1015–1025.
Gozlan, H., Laporte, A., Thibault, L., Schechter, L., Bolaños, F., and Hamon, M. 1994. Differential effects of N-Ethoxycarbonil-2-ethoxy-1,2-dihydroquinoline (EEDQ) on various 5-HT receptor binding sites in the rat brain. Neuropharmacology 33:423–431.
Henry, J. and Roth, G. 1984. Effect of aging on recovery of striatal dopamine receptors following N-ethoxycarbonyl-2-ethoxy-1, 2-dihydroquinoline (EEDQ) blockade. Life Sci. 35:899–904.
Adler, C., Meller, E., and Goldstein, M. 1985. Recovery of alpha 2-adrenoceptor binding and function after irreversible inactivation by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ). Eur. J. Pharmacol. 116:175–178.
Neve, K. and Molinoff, P. 1986. Turnover of beta 1-and beta 2-adrenergic receptors after down regulation or irreversible blockade. Mol. Pharmacol. 30:104–111.
Norman, A. and Creese, I. 1986. Effects of in vivo and in vitro treatments with N-ethoxy carbonyl-2-ethoxy-1,2-dihidroquinoline on putative muscarinic receptor subtypes in rat brain. Mol. Pharmacol. 30:96–103.
Nowak, G. 1990. Measurement of the receptor turnover using an irreversible inactivating method. Theoretical assumptions, limitations and practical realization. Polish J. Pharmacol. Pharmacy 42:433–440.
Bolaños, F., Schechter, L., Laporte, A., Hamon, M., and Gozlan, H. 1991. Recovery of 5-HT1A receptors after irreversible blockade by N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ). Proc. West. Pharmacol. Soc. 34:387–393.
Raymond, J., Mukhin, Y., Gettys, T., and Garnovskaya, M. 1999. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 127:1751–1764.
De Vivo, M. and Maayani, S. 1986. Characterization of 5-HT1A receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in guinea pig and rat hippocampal membranes. J. Pharmacol. Exp. Ther. 238:248–253.
Zgombick, J., Beck, S., Mahle, C., Craddock-Royal, I. B., and Maayani, S. 1989. Pertussis toxin-sensitive guanine nucleotidebinding protein(s) couple adenosine A1 and 5-hydroxytryptamine1A receptors to the same effector systems in rat hippocampus. Biochemical and physiological studies. Mol. Pharmacol. 35:484–494.
Shenker, A., Maayani, S., Weinstein, H., and Green, J. 1987. Pharmacological characterisation of two 5-hydroxytryptamine receptors coupled to adenylate cyclase in guinea-pig hippocampal membranes. Mol. Pharmacol. 31:357–367.
Markstein, R., Matsumoto, M., Kohler, C., Togashi, H., Yoshioka, M., and Hoyer, D. 1999. Pharmacological charcaterisation of 5-HT receptors positively coupled to adenylyl cyclase in the rat hippocampus. Naunyn-Schmideberg's Arch. Pharmacol. 359:454–459.
Claustre, Y., Benavides, J., and Scatton, B. 1988. 5-HT1A receptor agonists inhibit carbachol-induced stimulation of phosphoinositide turnover in rat hippocampus. Eur. J. Pharmacol. 149:149–153.
Pennington, N. and Kelly, J. 1990. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron 4:751–758.
Ishizuka, J., Beauchamp, R., Townsend, C., Greeley, G., and Thompson, J. 1992. Receptor-mediated autocrine growth-stimulatory effect of 5-hydroxytryptamine on cultured human pancreatic carcinoid cells. J. Cell Physiol. 150:1–7.
Lauder, J. 1993. Neurrotransmitters as growth signals: role of receptors and second messengers. Trends Neurosci. 16:233–240.
Whittaker-Azmitia, P. 1991. Role of serotonin and other neurotransmitter receptors in brain development. Basis for developmental pharmacology. Pharmacol. Rev. 43:347–352.
Mattson, M., Taylor-Hunter, A., and Kater, S. 1988. Neurite outgrowth in individual neurons of a neuronal population is differentially regulated by calcium and cyclic AMP. J. Neurosci. 8:1704–1711.
Murrain, M., Don Murphy, A., Mills, L., and Kater, S. 1990. Neuron-specific modulation by serotonin of regenerative outgrowth and intracellular calcium within the CNS of Helisoma trivolis. J. Neurobiol. 21:611–618.
Sikich, L., Hickok, J., and Todd, R. 1990. 5-HT1A receptors control neurite branching during development. Dev. Brain. Res. 56:269–274.
Urbina, M., Schmeer, C., and Lima, L. 1996. 5-HT1A receptor agonist differentially increases cyclic AMP concentration in intact and lesioned goldfish retina. In vitro inhibition of outgrowth by forskolin. Neurochem. Int. 29:453–460.
Lowry, O., Rosebrough, N., Farr, A., and Randall, R. 1951. Protein measurements with folin phenol reagent. J. Biol. Chem. 193:265–275.
Roth, B., Craigo, S., Choudhary, S., Uluer, A., Monsma, F., Shen, Y., Meltzer H., and Sibley, D. 1994. Binding of typical and atypical antipsychotic agents to 5-hidroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharm. Exp. Ther. 268:1403–1410.
Lima, L., Matus, O., and Drujan, B. 1988. Taurine effect on neuritic growth from goldfish retinal explants. Int. J. Devl. Neurosci. 6:417–424.
Sladeczek, F. and Bockaert, J. 1983. Turnover in vivo of alpha 1-adrenergic receptors in rat submaxillary glands. Mol. Pharmacol. 23:282–288.
Barlow, R. 1983. Biodata handling with microcomputers. Amsterdam: Elsevier.
Pedigo, N., Yamamura, H., and Nelson, D. 1981. Discrimination of multiple [3H]5-hydroxytryptamine binding sites by the neuroleptic spiperone in rat brain. J. Neurochem. 36:220–226.
Hamon, M., Bourgoin, S., Gozlan, H., Hall, M., Goetz, C., Artaud, F., and Horn, A. (1984) Biochemical evidence for the 5-HT agonist properties of PAT (8-hydroxy-2-(di-n-propylamino) tetralin) in the rat brain. Eur. J. Pharmacol. 100:263–276.
Ieni, J. and Meyerson, L. 1988. The 5-HT1A receptor probe [3H]8-OH-DPAT labels the 5-HT transporter in human platelets. Life Sci. 42:311–320.
Nénonéné, E., Radja, F., Carli, M., van Gelder, N., Afkhami-Dastjerdian, S., and Reader, T. 1996. Alkylation of [3H]8-OHDPAT binding sites in rat.
Eglen, R., Jasper, J., Chang, D., and Martin, G. 1997. The 5-HT7 receptor: orphan found. TiPS 18:104–107.
Ying, S.-W. and Rusak, B. 1997. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. 755:246–254.
Chidlow, G., Le, Corre, S., and Osborne, N. 1998. Localization of 5-hydroxytryptamine1A and 5-hydroxytryptamine7 receptors in rabbit ocular and brain tissues. Neuroscience. 87:675–689.
Lanfumey, L., Haj-Dahmane, S., and Hamon, M. 1983. Further assesment of the antagonist properties of the novel and selective 5-HT1A receptor ligands (+)-WAY 100,135 and SDZ 216-525. Eur. J. Pharmacol. 249:25–35.
Pinto, W. and Battaglia, G. 1993. In vivo EEDQ dose-dependently inactivate rat brain 5-HT receptors but not 5-HT uptake sites. NeuroReport. 5:61–64.
Kettle, C., Cheetham, S., Martin, K., Prow, M., and Heal, D. 1999. The effects of the peptide-coupling agent, EEDQ, on 5-HT2A receptor binding and function in rat frontal cortex. Neuropharmacology 38:1421–1430.
Belleau, B., Di, Ttullio V., and Godin, D. 1969. The mechanism of irreversible adrenergic blockade by N-carbethoxydihydroquinolines. Model studies with typical serine hydrolases. Biochem. Pharmacol. 18:1039–1044.
Keck, B. and Lakoski, J. 1996. Region-specific serotonin1A receptor turnover following irreversible blockade with EEDQ. NeuroReport 7:2717–2721.
Rydel, R. and Greene, L. 1988. cAMP analogs promote survival and neurite outgrowth in cultures of rat sympathetic and sensory neurons independently of nerve growth factor. Proc. Natl. Acad. Sci. USA 85:1257–1261.
Wang, Q. and Zheng, J. 1998. cAMP-mediated regulation of neurotrophin-induced collapse of nerve growth cones. J. Neurosci. 18:4973–4984.
Ekstrom, P. 1995. Increased cyclic AMP in in vitro regenerating frog sciatic nerves inhibits Schwann cell proliferation but has no effect on axonal outgrowth. J. Neurosci. Res. 42:54–62.
Osborne, N. 1990. Effects of GTP, forskolin, sodium fluoride, serotonin, dopamine, and carbachol on adenylate cyclase in Teleost retina. Neurochem. Res. 15:523–528.
Kudo, Y. and Boyd, C. A. R. 2000. Heterodimeric amino acid transpòrters:expression of heavy but not light chains of CD98 correlates with the induction of amino acid transport systems in human placental trophoblast. J. Physiol. 523:13–18.
Lippe, C. and Ardizzone, C. 1990 Actions of vasopressin and isoprenaline on the ionic transport across the isolated frog skin in the presence and the absence of adenyl cyclase inhibitors MDL12330A and SQ22536. Comp. Biochem. Physiol. 99C: 209–211.
Lima, L., Matus, P., and Urbina, M. 1994. Serotonin inhibits outgrowth of goldfish retina and impairs the trophic effect of taurine. J. Neurosci. Res. 38:444–450.
Riad, M., Emerit, M. B., and Hamon, M. 1994. Neurotrophic effects of ipsapirone and other 5-HT1A receptor agonists on septal cholinergic neurons in culture. Brain Res. Dev. Brain Res. 82:245–258.
Gadea, A., López, E., and López-Colomé, A. 1999. The adenylate cyclase inhibitor MDL-12330A has a non-specific effect on glycine transport in Mũller cells from the retina. Brain Res. 838:200–204.
Braumann, T., Jastorff, B., and Richter-Landsberg, C. 1986. Fate of cyclic nucleotides in PC12 cell cultures: uptake, metabolism, and effects of metabolites on nerve growth factor-induced neurite outgrowth. J. Neurochem. 47:912–919.
Hamon, M., Gozlan, H., El, Mestikawy, S., Emerit, M., Bolaños, F., and Schechter, L. 1990. The central 5-HT1A receptors: pharmacological, biochemical, functional and regulatory properties. Ann. NY Acad. Sci. 600:114–131.
Markstein, R., Hoyer, D., and Engel, G. 1986 5-HT1A-receptors mediate stimulation of adenylate cyclase in rat hippocampus. Naunyn-Schmiedeberg's Arch. Pharmacol. 333:335–341.
Thomas, D., Middlemiss, D., Taylor, S., Nelson, P., and Brown, A. 1999. 5-CT stimulation od adenylyl cyclase activity in guinea-pig hippocampus: evidence for involvement of 5-HT7 and 5-HT1A receptors. Br. J. Pharmacol. 128:158–164.
Cadogan, A., Kendall, D., and Marsden, C. 1994. Serotonin 5-HT1A receptor activation increases cyclic AMP formation in the rat hippocampus in vivo. J. Neurochem. 62:1816–1821.
Osborne, N. and Ghazi, H. 1991. 5-HT1A receptors positively coupled to cAMP formation in the rabbit retina. Neurochem. Int. 19:407–411.
Okada, F., Tokumitsu, Y., and Nomura, Y. 1989. Pertussis toxin attenuates 5-hydroxytryptamine1A receptor-mediated inhibition of forskolin-stimulated adenylate cyclase activity in rat hippocampal membranes. J. Neurochem. 52:1566–1569.
Hamon, M. 1997. Seotoninergic Neurons and 5-HT Receptors in the CNS. Handbook of Experimental Pharmacology, vol. 129. Edited by H. G. Baumgarten and M. Göthert, Springer-Verlag.
Law, S. and Reisine, T. 1997. Changes in the association of G protein subunits with the cloned mouse delta opioid receptor on agonist stimulation. J. Pharmacol. Exp. Ther. 281:476–1486.
Federman, A., Conklin, B., Schrader, K., Reed, R., and Bourne, H. 1992. Hormonal stimulation of adenylyl cyclase through Giprotein bg subunits. Nature 356:59–161.
Albert, P., Morris, S., Ghahremani, M., Storring, J., and Lembo P. 1998. A putative alpha-helical G beta gamma-coupling domain in the second intracellular loop of the 5-HT1A receptor. Ann. NY Acad. Sci. 861:146–161.
Liu, Y., Ghahremani, M., Rasenick, M., Jakobs, K., and Albert, P. 1999. Stimulation of cAMP synthesis by Gi-coupled receptors upon ablation of distinct Gai protein expression. Gi subtype specificity of the 5-HT1A receptor. J. Biol. Chem. 274:16444–16450.
Albert, P., Sajedi, N., Lemonde, S., and Ghahremani, M. 1999. Constitutive G(i2)-dependent activation of adenylyl cyclase type II by the 5-HT1A receptor. Inhibition by anxiolytic partial agonists. J. Biol. Chem. 274:35469–35474.
Middleton, J., Raymond, J., Whorton, A., and Dennis, V. 1990. Short-term regulation of Na+/K+ adenosine triphosphate by recombinant human serotonin 5-HT1A receptor expressed in HeLa cells. J. Clin. Invest. 86:1799–1805.
Hurley, J. 1999. Structure, mechanism, and regulation of mammalian adenylyl cyclase. J. Biol. Chem. 274:7599–7602.
Nash, M., Wood, J., and Osborne, N. 1997. Protein kinase C activation by serotonin potentiates agonist-induced stimulation of cAMP production in cultured rat retinal pigment epithelial cells. Exp. Eye Res. 64:249–55.
Kirkham, D. and Henley, J. 1993. Characterization of adenylyl cyclase in goldfish brain. Biochem. Pharmacol. 46:1559–1563.
Kirkham, D. and Henley, J. 1994. Characteristics of the goldfish brain adenylyl cyclase system. Biochem. Soc. Trans. 22: 227S.
Simonds, W. 1999. G protein regulation of adenylate cyclase. Trends Pharmacol. Sci. 20:66–73.
Gao, B. and Gilman, A. 1991. Cloning and expression of a widely distributed (type IV) adenylyl cyclase. Proc. Natl. Acad. Sci. USA 88:10178–10182.
Xia, Z., Choi, E., Wang, F., Blazynski, C., and Storm, D. 1993. Type I calmodulin-sensitive adenylyl cyclase is neural specific. J. Neurochem. 60:305–311.
Beitz, E., Völkel, H., Guo, Y., and Schultz J. 1998. Adenylyl cyclase type 7 is the predominant isoform in the bovine retinal pigment epithelium. Acta Anatomica 162:157–162.
Watson, P., Kuprinski, A., Kempinski, C., and Frankenfield, C. 1994. Molecular cloning and characterization of the type VII isoform of mammalian adenylyl cyclase expressed widely in mouse and in S49 mouse lymphoma cells. J. Biol. Chem. 269:28893–28898.
Goldberg, J., Mills, L., and Kater, S. 1992. Effects of serotonin on intracellular calcium in embryonic and adult Helisoma neurons. Int. J. Dev. Neurosci. 10:255–264.
Lima, L., Matus, P., and Drujan, B. 1993. Taurine-induced regeneration of goldfish retina in culture may involve a calciummediated mechanism. J. Neurochem. 60:2153–2158.
Cooper, R., Fernández de, Miguel F., Adams, W., and Nicholls, J. 1991. Anterograde and retrograde effects of synapse formation on calcium currents and neurite outgrowth in cultured leech neurons. Proc. R. Soc. Lond. 249:217–222.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schmeer, C., Obregón, F., Urbina, M. et al. Further Characterization of 5-HT1A Receptors in the Goldfish Retina: Role of Cyclic AMP in the Regulation of the In Vitro Outgrowth of Retinal Explants. Neurochem Res 26, 213–223 (2001). https://doi.org/10.1023/A:1010960332229
Issue Date:
DOI: https://doi.org/10.1023/A:1010960332229