Abstract
Mutations in myelin protein zero (P0) are responsible for several peripheral neuropathies. We studied transport and membrane integration of the truncated P0 mutants using transfected oligodendroglial cell line (Oln93). Starting with rat cDNA, we produced two P0 deletions. The first, called P0-Tyr contains a 66 amino acid deletion in the extracellular domain and a tyrosine at the new position 32. In the second, called P0-Cys, the tyrosine 32 is replaced by a cysteine. This replacement restores a disulfide bond in the extracellular domain. Our results show that P0 proteins, truncated or not, were expressed in the plasma membrane of the transfected cells. Transcription rates of both mutants were normal. However, P0-Tyr was detected in only 3-5% of the cells compared to the P0-Cys and the wild type. Thus, the disulfide bond in the extracellular domain is important for stability and correct addressing of the P0 protein.
Similar content being viewed by others
REFERENCES
Lemke, G. and Axel, R. 1985. Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin. Cell 40:501–508.
Lemke, G., Lamar, E., and Patterson, J. 1988. Isolation and analysis of the gene encoding peripheral myelin protein zero. Neuron 1:73–83.
Williams, A. F. and Barclay, A. N. 1988. The immunoglobulin superfamily-domains for cell surface recognition. Annu. Rev. Immunol. 6:381–405.
Greenfield, S., Brostoff, S., Eylar, E. H., and Morell, P. 1973. Protein composition of myelin of the peripheral nervous system. J. Neurochem. 20:1207–1216.
Kirschner, D. A. and Ganser, A. L. 1980. Compact myelin exists in the absence of basic protein in the shiverer mutant mouse. Nature 283:207–210.
Ding, Y. and Brunden, K. R. 1994. The cytoplasmic domain of myelin glycoprotein P0 interacts with negatively charged phospholipid bilayers. J. Biol. Chem. 269:10764–10770.
Filbin, M. T. and Tennekoon, G. I. 1990. High level of expression of the myelin protein P0 in Chinese hamster ovary cells. J. Neurochem. 55:500–505.
D'Urso, D., Brophy, P. J., Staugaitis, S. M., Gillespie, C. S., Frey, A. B., Stempak, J. G., and Colman, D. R. 1990. Protein zero of peripheral nerve myelin: biosynthesis, membrane insertion, and evidence for homotypic interaction. Neuron 4:449–460.
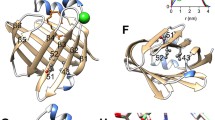
Shapiro, L., Doyle, J. P., Hensley, P., Colman, D. R., and Hendrickson, W. A. 1996. Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin. Neuron 17:435–449.
Schneider-Schaulies, J., von Brunn, A., and Schachner, M. 1990. Recombinant peripheral myelin protein P0 confers both adhesion and neurite outgrowth-promoting properties. J. Neurosci. Res. 27:286–297.
Zhang, K. and Filbin, M. T. 1994. Formation of a disulfide bond in the immunoglobulin domain of the myelin P0 protein is essential for its adhesion. J. Neurochem. 63:367–370.
Filbin, M. T. and Tennekoon, G. I. 1993. Homophilic adhesion of the myelin P0 protein requires glycosylation of both molecules in the homophilic pair. J. Cell Biol. 122:451–459.
Filbin, M. T., Zhang, K., Li, W., and Gao, Y. 1999. Characterization of the effect on adhesion of different mutations in myelin P0 protein. Ann. NY Acad. Sci. 883:160–167.
Nelis, E., Haites, N., and Van Broeckhoven, C. 1999. Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies. Hum. Mutat. 13:11–28.
Friedrich, V. L. Jr., Holstein, G. R., Li, X., Gow, A., Kelley, K. A., and Lazzarini, R. A. 1993. Intracellular distribution of transgenic bacterial beta-galactosidase in central nervous system neurons and neuroglia. J. Neurosci. Res. 36:88–98.
Richter-Landsberg, C. and Heinrich, M. 1996. OLN-93: a new permanent oligodendroglia cell line derived from primary rat brain glial cultures. J. Neurosci. Res. 45:161–173.
Lennette, D. A. 1978. An improved mounting medium for immunofluorescence microscopy [letter]. Am. J. Clin. Pathol. 69:647–648.
Shaw, P., Freeman, J., Bovey, R., and Iggo, R. 1996. Regulation of specific DNA binding by p53: evidence for a role for O-glycosylation and charged residues at the carboxy-terminus. Oncogene 12:921–930.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
Zhang, K. and Filbin, M. T. 1998. Myelin P0 protein mutated at cys21 has a dominant-negative effect on adhesion of the wild type P0. J. Neurosci. Res. 53:1–6.
Deschenes, S. M., Walcott, J. L., Wexler, T. L., Scherer, S. S., and Fischbeck, K. H. 1997. Altered trafficking of mutant connexin32. J. Neurosci. 17:9077–9084.
Hoffmann, C., Moro, S., Nicholas, R. A., Harden, T. K., and Jacobson, K. A. 1999. The role of amino acids in extracellular loops of the human P2Y1 receptor in surface expression and activation processes. J. Biol. Chem. 274:14639–14647.
Fan, G. F., Ray, K., Zhao, X. M., Goldsmith, P. K., and Spiegel, A. M. 1998. Mutational analysis of the cysteines in the extracellular domain of the human Ca2+ receptor: effects on cell surface expression, dimerization and signal transduction. FEBS Lett. 436:353–356.
Marchand, P., Volkmann, M., and Bond, J. S. 1996. Cysteine mutations in the MAM domain result in monomeric meprin and alter stability and activity of the proteinase. J. Biol. Chem. 271:24236–24241.
Hurtley, S. M. and Helenius, A. 1989. Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol. 5:277–307.
Klausner, R. D. 1989. Architectural editing: determining the fate of newly synthesized membrane proteins. New Biol. 1:3–8.
Hammond, C. and Helenius, A. 1995. Quality control in the secretory pathway. Curr. Opin. Cell Biol. 7:523–529.
Wickner, S., Maurizi, M. R., and Gottesman, S. 1999. Post-translational quality control: folding, refolding, and degrading proteins. Science 286:1888–1893.
Klausner, R. D. and Sitia, R. 1990. Protein degradation in the endoplasmic reticulum. Cell 62:611–614.
Bonifacino, J. S. and Lippincott-Schwartz, J. 1991. Degradation of proteins within the endoplasmic reticulum. Curr. Opin. Cell Biol. 3:592–600.
Mellman, I. 1996. Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12:575–625.
Coux, O., Tanaka, K., and Goldberg, A. L. 1996. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65:801–847.
Mitch, W. E. and Goldberg, A. L. 1996. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. New Engl. J. Med. 335:1897–1905.
Cheng, S. H., Gregory, R. J., Marshall, J., Paul, S., Souza, D. W., White, G. A., O'Riordan, C. R., and Smith, A. E. 1990. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 63:827–834.
Sifers, R. N., Finegold, M. J., and Woo, S. L. 1992. Molecular biology and genetics of alpha 1-antitrypsin deficiency. Semin. Liver Dis. 12:301–310.
Copeland, C. S., Doms, R. W., Bolzau, E. M., Webster, R. G., and Helenius, A. 1986. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J. Cell Biol. 103: 1179–1191.
Zhang, J. X., Braakman, I., Matlack, K. E., and Helenius, A. 1997. Quality control in the secretory pathway: the role of calreticulin, calnexin and BiP in the retention of glycoproteins with C-terminal truncations. Mol. Biol. Cell 8:1943–1954.
Thomas, P. J., Qu, B. H., and Pedersen, P. L. 1995. Defective protein folding as a basis of human disease. Trends Biochem. Sci. 20:456–459.
Carrell, R. W., Lomas, D. A., Sidhar, S., and Foreman, R. 1996. Alpha 1-antitrypsin deficiency. A conformational disease. Chest 110:243S–247S.
Beissinger, M. and Buchner, J. 1998. How chaperones fold proteins. Biol. Chem. 379:245–259.
Bross, P., Corydon, T. J., Andresen, B. S., Jorgensen, M. M., Bolund, L., and Gregersen, N. 1999. Protein misfolding and degradation in genetic diseases. Hum. Mutat. 14:186–198.
Gregersen, N., Bross, P., Jorgensen, M. M., Corydon, T. J., and Andresen, B. S. 2000. Defective folding and rapid degradation of mutant protein is a common disease mechanism in genetic disorders. J. Inherit. Metab. Dis. 23:441–447.
Naef, R., Adlkofer, K., Lescher, B., and Suter, U. 1997. Aberrant protein trafficking in Trembler suggests a disease mechanism for hereditary human peripheral neuropathies. Mol. Cell Neurosci. 9:13–25.
D'Urso, D., Prior, R., Greiner-Petter, R., Gabreels-Festen, A. A., and Muller, H. W. 1998. Overloaded endoplasmic reticulum-Golgi compartments, a possible pathomechanism of peripheral neuropathies caused by mutations of the peripheral myelin protein PMP22. J. Neurosci. 18:731–740.
Tobler, A. R., Notterpek, L., Naef, R., Taylor, V., Suter, U., and Shooter, E. M. 1999. Transport of Trembler-J mutant peripheral myelin protein 22 is blocked in the intermediate compartment and affects the transport of the wild-type protein by direct interaction. J. Neurosci. 19:2027–2036.
Bruzzone, R., White, T. W., Scherer, S. S., Fischbeck, K. H., and Paul, D. L. 1994. Null mutations of connexin32 in patients with X-linked Charcot-Marie-Tooth disease. Neuron 13:1253–1260.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pfend, G., Matthieu, JM., Garin, N. et al. Implication of the Extracellular Disulfide Bond on Myelin Protein Zero Expression. Neurochem Res 26, 503–510 (2001). https://doi.org/10.1023/A:1010908828134
Issue Date:
DOI: https://doi.org/10.1023/A:1010908828134