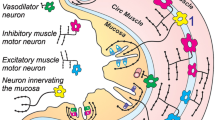
Abstract
The distribution of nitric oxide synthase in both neuronal and non-neuronal pancreatic tissues and the role of nitric oxide in the control of exocrine pancreatic secretion are reviewed in this article. Earlier reports based on in vivo studies suggested that nitric oxide can affect the secretory activity of the exocrine pancreas through changes in pancreatic blood flow. More recently, the employment of either nitric oxide synthase inhibitors or nitric oxide donors in in vitro preparations has provided evidence that nitric oxide can exert a direct action on this gland independently on its vascular effects. Most research in this area seems to indicate that modulation of exocrine pancreatic function by nitric oxide is exerted via activation of guanylate cyclase and generation of cGMP, although other pathways cannot be excluded. Experiments performed over the last year in our laboratory reveal a novel and interesting mechanism based on the ability of nitric oxide to control the release of endogenous neurotransmitter in the pancreas and, subsequently, the nerve-mediated enzyme secretion.
Similar content being viewed by others
References
Furchgott RF, Zawadzki JV: The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980
Palmer RMJ, Ferrige AG, Moncada S: Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327: 524–526, 1987
Wilcox CS, Welch WJ, Murad F, Gross SS, Taylor G, Levi R, Schmidt HH: Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci USA 89: 11993–11997, 1992
Schmidt HH, Warner TD, Ishii K, Scheng H, Murad F: Insulin secretion from b-pancreatic cells caused by L-Arginine-derived nitrogen oxides. Science 258: 1376–1378, 1992
Nakane M, Schmidt HH, Pollock SJ, Förstermann U, Murad F: Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett 316: 175–180, 1993
Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS: Nitric oxide synthase in human and rat lung: Immunocytochemical and immunohistochemical localization. Am J Respir Cell Mol Biol 9: 371–377, 1993
Schmidt HH, Gagne GD, Nakane M, Pollock JS, Miler MF, Murad F: Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem 40: 1439–1456, 1992
Stuehr DJ, Griffith OW: Mammalian nitric oxide synthases. Adv Enzymol 65: 287–346, 1992
Busse R, Mülsch A: Induction of nitric oxide synthase by cytokines in vascular smooth muscle cells. FEBS Lett 275: 87–90, 1990
Hibbs JB Jr, Taintor RR, Vavrin Z, Rachlin EM: Nitric oxide: A cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun 157: 87–94, 1988
Wright DE, Mülsch A, Busse R, Osswald H: Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun 160: 813–819, 1989
Billiar TR, Curran RD, Stuehr DJ, Stadler J, Simmons RL, Murray SA: Inducible cytosolic enzyme activity for the production of nitrogen oxides from L-Arginine in hepatocytes. Biochem Biophys Res Commun 168: 1034–1040, 1990
Radomski MW, Palmer RMJ, Moncada S: Glucocorticoids inhibit the expression of an inducible but not the constitutive nitric oxide synthase in vascular endothelial cells. Proc Natl Acad Sci USA 87: 10043–10047, 1990
Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C: Calmodulin as a tightly bound subunit of calcium-, calmodulin-independent nitric oxide synthase. J Exp Med 176: 599–604, 1992
Stark ME, Szurszewski JH: Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology 103: 1928–1949, 1992
Kilbourn RG, Griffith OW: Over-production of nitric oxide in cytokine-mediated and septic shock. J Natl Cancer Inst 84: 1671–1672, 1992
Nowicki JP, Duval D, Poignet H, Scatton B: Nitric oxide mediates neuronal death after local cerebral ischemia. Eur J Pharmacol 204: 339–340, 1991
Schaad NC, Vanecek J, Schulz PE: Photoneural regulation of rat pineal nitric oxide synthase. J Neurochem 62: 2496–2499, 1994
Konturek SK, Konturek PC: Role of nitric oxide in the digestive system. Digestion 56: 1–13, 1995
Stamler JS, Singel DJ, Loscalzo J: Biochemistry of nitric oxide and its redox-activated forms. Science 258: 1898–1902, 1992
Feelisch M, te Poel M, Zamora R, Deussen A, Moncada S: Understanding the controversy over the identity of endothelium-derived relaxing factor. Nature 368: 62–65, 1994
Lonart G, Johnson KM: Characterization of nitric oxide generator-induced hippocampal 3H-norepinephrine release: II. The role of calcium, reverse norepinephrine transport and cyclic 3′5′-guanosine monophosphate. J Pharmacol Exp Ther 275: 14–22, 1995
Wiklund CU, Olgart C, Wiklund NP, Gustafsson LE: Modulation of cholinergic and substance P-like neurotransmission by nitric oxide in the guinea-pig ileum. Br J Pharmacol 110: 833–839, 1993
Feelisch M: The biochemical pathways of nitric oxide formation from nitrovasodilators: Appropriate choice of exogenous NO donors and aspects of preparation and handling of aqueous NO solutions. J Cardiovasc Pharmacol 17: S25–S33, 1991
Salomons H, Keaveny AP, Henihan R, Offner G, Sengupta A, Lamorte WW, Afdhal NH: Nitric oxide and gallbladder motility in prairie dogs. Am J Physiol 272: G770–G778, 1997
Prast H, Tran MH, Fischer H, Philippu A: Nitric oxide-induced release of acetylcholine in the nucleus accumbens: Role of cyclic GMP, glutamate, and GABA. J Neurochem 71: 266–273, 1998
Suzuki T, Nakajima K, Fujimoto K, Fujii T, Kawashima K: Nitric oxide increases stimulation-evoked acetylcholine release from rat hippocampal slices by a cyclic GMP-independent mechanism. Brain Res 760: 158–162, 1997
Olken NM, Marletta MA: NG-methyl-L-Arginine functions as an alternate substrate and mechanism-based inhibitor of nitric oxide synthase. Biochemistry 32: 9677–9685, 1993
Furfine ES, Harmon MF, Paith JE, Garvey EP: Selective inhibition of constitutive nitrergic oxide synthase by L-NG-nitroarginine. Biochemistry 32: 8512–8517, 1993
Lambert LE, Whitten JP, Baron BM, Cheng HC, Doherty NS, McDonal IA: Nitric oxide synthesis in the central nervous system, endothelium, and macrophages differs in its sensitivity to inhibition by arginine analogues. Life Sci 48: 69–75, 1991
Bogle RG, Moncada S, Pearson JD, Mann GE: Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-Arginine transporter. Br J Pharmacol 105: 768–770, 1992
Rand MJ, Li CG: The inhibition of nitric oxide-mediated relaxations in rat aorta and anococcygeous muscle by diphenylene iodonium. Clin Exp Pharmacol Physiol 20: 141–148, 1993
Babbedge RC, Bland-Ward PA, Moore PK: Inhibition of rat cerebellar nitric oxide synthase by 7-nitro-indazole and related substituted indazoles. Br J Pharmacol 110: 225–228, 1993
Umans GJ, Samsel RW: L-Canavanine selectively augments contraction in aorta from endotoxemic rats. Eur J Pharmacol 210: 343–346, 1992
Misko TP, Moore WM, Kasten TP, Nickols GA, Corbett JA, Tilton RG, McDaniel ML, Williamson JR, Currie MG: Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol 233: 119–125, 1993
Doyle MP, Hockstra JW: Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J Inorg Biochem 14: 351–358, 1981
Saran M, Michel C, Bors W: Reaction of nitric oxide with O2 -. Implications for the action of endothelium-derived relaxing factor. Free Rad Res Commun 10: 221–226, 1990
Rubanyi GM, Vanhoutte PM: Superoxide anion and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 250: H822–H827, 1986
Chakder S, Rattan S: Neurally mediated relaxation of opossum internal anal sphincter: Influence of superoxide anion generator and the scavenger. J Pharmacol Exp Ther 260: 1113–1118, 1992
Blanquet F, Abysique A, Gonella J: In vivo study of the role of muscarinic receptors in the parasympathetic control of rabbit colonic motility. J Auton Nerv Syst 46: 217–227, 1994
Boeckxstaen GE, De Man JG, Pelckmans PA, Herman AG, Van Maercke YM: α2-adrenoceptor-mediated modulation of the nitrergic innervation of the canine isolated ileocolonic junction. Br J Pharmacol 109: 1079–1084, 1993
Assreuy J, Cunha FQ, Liew FY, Moncada S: Feedback inhibition of nitric oxide synthase activity by nitric oxide. Br J Pharmacol 108: 833–837, 1993
Tremblay J., Gerzer R., Hamet P: Cyclic GMP in cell function. In: P. Greengard, G.A. Robison (eds). Advances in Second Messenger and Phosphoprotein Research. Raven Press, New York, 1988, pp 319–384
Yoshida H, Tsunoda Y, Owyang C: Effect of uncoupling NO/cGMP pathways on carbachol-and CCK-stimulated Ca2+ entry and amylase secretion from the rat pancreas. Eur J Physiol 434: 25–37, 1997
Gibson A, Babbedge R, Brave SR, Hart SL, Hobbs AJ, Tucker JF, Wallace P, Moore PK: An investigation of some S-nitrosothiols, and hydroxyarginine, on the mouse anococcygeous. Br J Pharmacol 107: 715–721, 1992
Rapoport RM, Murad F: Effects of ethacrynic acid and cystamine on sodium nitroprusside-induced relaxation, cyclic GMP levels, and guanylate cyclase activity in rat aorta. Gen Pharmacol 19: 61–65, 1983
Xu X, Star RA, Tortorici G, Muallem S: Depletion of intracellular calcium stores activates nitric oxide synthase to generate cGMP and regulate calcium influx. J Biol Chem 269: 12645–12653, 1994
Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B: Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,4,a]quioxalin-1-one. Mol Pharmacol 48: 184–188, 1995
Furchgott RF, Jothianandan D: Endothelium-dependent and-independent vasodilation involving cyclic GMP: Relaxation induced by nitric oxide, carbon monoxide, and light. Blood Vess 28: 52–61, 1991
Marczin N, Ryan US, Catravas JD: Methylene blue inhibits nitrovasodilator-and endothelium-derived relaxing factor-induced cyclic GMP accumulation in cultured pulmonary arterial smooth muscle cells via generation of superoxide anion. J Pharmacol Exp Ther 263: 170–179, 1992
Gukovskaya A, Pandol S: Nitric oxide production regulates cGMP formation and calcium influx in pancreatic acinar cells. Am J Physiol 266: G350–G356, 1994
Mirzazadeh S, Hobbs AJ, Tucker JF, Gobson A: Cyclic nucleotide content of the rat anococcygeous during relaxations induced by drugs or by nonadrenergic noncholinergic field stimulation. J Pharm Pharmacol 43: 247–257, 1991
Hebeiss K, Kilbinger H: Nitric oxide-sensitive guanylyn cyclase inhibits acetylcholine release and excitatory motor transmission in the guinea-pig ileum. Neuroscience 82: 623–629, 1998
Buxton IL, Cheek DJ, Exkman D, Westfall DP, Sanders KM, Keef KD: NG-nitro-L-Arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res 72: 387–395, 1993
Hope BT, Vincent SRAD: Histochemical characterization of neuronal NADPH-diaphorase. J Histochem Cytochem 37: 653–661, 1989
Adeghate E, Donath T: Distribution of neuropeptide Y and vasoactive intestinal polypeptide immunoreactive nerves in normal and transplanted pancreatic tissue. Peptides 11: 1087–1092, 1990
Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH: Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA 88: 7797–7801, 1991
Ekblad E, Alm P, Sundler F: Distribution, origin and projections of nitric oxide synthase-containing neurons in gut and pancreas. Neuroscience 63: 233–248, 1994
Umehara K, Kataoka K, Ogura T, Esumi H, Kashima K, Ibata Y, Okamura H: Comparative distribution of nitric oxide synthase (NOS) in pancreas of the dog and rat: Immunocytochemistry of neuronal type NOS and histochemistry of NADPH-diaphorase. Brain Res Bull 42: 649–678, 1997
Liu HP, Tay SS, Leong SK: Nitrergic neurons in the pancreas of newborn guinea-pig: Their distribution and colocalization with various neuropeptides and dopamine-beta-hydroxylase. J Auton Nerv Syst 61: 248–256, 1996
Tay SS, Moules EW, Burnstock G: Colocalisation of NADPH-diaphorase with nitric oxide synthase and vasoactive intestinal polypeptide in newborn pancreatic neurons. J Anat 184: 545–552, 1994
Worl J, Wiesand M, Mayer B, Greskotter KR, Neuhuber WL: Neuronal and endothelial nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in rat and human pancreas: influence of fixation. Histochem 102: 353–364, 1994
Burrel MA, Montuenga LM, Garcia M, Villaro AC: Detection of nitric oxide synthase (NOS) in somatostatin-producing cells of human and murine stomach and pancreas. J Histochem Cytochem 44: 339–346, 1996
Shimosegawa T, Abe T, Satoh A, Abe R, Kikuchi Y, Koizumi M, Toyota T: NADPH-diaphorase activity in neurons of the mammalian pancreas: coexpression with vasoactive intestinal popypeptide. Gastroenterology 105: 999–1008, 1993
Liu HP, Leong SK, Tay SS: Localization of NADPH-diaphorase positive neurons in the pancreas of the mouse, rat, chick, kitten and monkey. J Hirnforsch 35: 501–510, 1994
Kirchgessner AL, Liu MT, Gershon MD: NADPH diaphorase (nitric oxide synthase)-containing nerves in the enteropancreatic innervation: Sources, co-stored neuropeptides, and pancreatic function. J Comp Neurol 342: 115–130, 1994
Ember ZS: Age-dependent distribution of nitric oxide synthase containing elements in the rat pancreas. Biogenic Amines 13: 277–284, 1997
Kugler P, Hofer D, Mayer B, Drenckhahn D: Nitric oxide synthase and NADP-linked glucose-6-phosphatase dehydrogenase are co-localized in brush cells of rat stomach and pancreas. J Histochem Cytochem 42: 1317–1321, 1994
Tay SS, Moules EW: NADPH-diaphorase is colocalized with nitric oxide synthase and vasoactive intestinal polypeptide in rat pancreatic neurons in culture. Arch Hist Cytol 57: 253–257, 1994
Furuzawa Y, Ohmori Y, Watanabe T: Immunohistochemical studies of neural elements in pancreatic islets of the cat. J Vet Med Sci 58: 641–646, 1996
Shimosegawa T, Abe T, Satoh A, Asakura T, Yoshida K, Koizumi M, Toyota T: Histochemical demonstration of NADPH-diaphorase activity, a marker for nitric oxide synthase, in neurons of the rat pancreas. Neurosci Lett 148: 67–70, 1992
Tay SS, Burnstock G: Localization of age-related changes in NADPH-diaphorase activity in pancreatic neurons. Neurosci 61: 597–602, 1994
Tharakan T, Kirchgessner AL, Baxi LV, Gershon MD: Appearance of neuropeptides and NADPH-diaphorase during development of the enteropancreatic innervation. Brain Res Dev Brain Res 84: 26–38, 1995
Berezin I, Snyder SH, Bredt DS, Daniel EEAD: Ultrastructural localization of nitric oxide synthase in canine small intestine and colon. Am J Physiol 266: C981–C989, 1994
Konturek SJ, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J: Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol 15: 19–28, 1994
Molero X, Guarner F, Salas A, Mourelle M, Puig V, Malagelada JR: Nitric oxide modulates pancreatic basal secretion and response to caerulein in the rat: Effects in acute pancreatitis. Gastroenterology 108: 1855–1862, 1995
Konturek JW, Hengst K, Kulesza E, Gabryelewicz A, Konturek SJ, Domschke W: Role of endogenous nitric oxide in the control of exocrine and endocrine pancreatic secretion in humans. Gut 40: 86–91, 1997
Patel AG, Toyama MT, Nguyen TN, Cohen GA, Ignarro LJ, Reber HA, Ashley SW: Role of nitric oxide in the relationship of pancreatic blood flow and exocrine secretion in cats. Gastroenterology 108: 1215–1220, 1995
Konturek SJ, Bilski J, Konturek PK, Cieszkowski M, Pawlik W: Role of endogenous nitric oxide in the control of canine pancreatic secretion and blood flow. Gastroenterology 104: 896–902, 1993
Maczka M, Thor P, Bilski J, Konturek SJ: Nitric oxide and the interrelation between intestinal motility and pancreatic secretion in fasted and fed dogs. J Physiol Pharmacol 45: 285–298, 1994
Satoh A, Shimosegawa T, Abe T, Kikuchi Y, Abe R, Koizumi M, Toyota T: Role of nitric oxide in the pancreatic blood flow response to caerulein. Pancreas 9: 574–579, 1994
Holst JJ, Rasmussen TN, Schmidt P: Role of nitric oxide in neurallyinduced pancreatic exocrine secretion in pigs. Am J Physiol 266: G206–G213, 1994
Wrenn RW, Currie MG, Herman LE: Nitric oxide participates in the regulation of acinar cell secretion. Life Sci 55: 511–518, 1994
Hernandez-Guijo JM, Acosta JJ, Garcia-Benito M, Lopez MA, San Roman JI, and Calvo JJ: Nitric oxide synthesis modulates amylase secretion after cholecystokinin stimulation in perfused pancreatic segments of the rat. Digestion 57: 236, 1996
Feelisch M, Noack EA: Correlation between nitric oxide formation during degradation of organic nitrates and activation of guanylate cyclase. Eur J Pharmacol 139: 19–30, 1987
Radomski MW, Palmer RMJ, Moncada S: An L-Arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc Natl Acad Sci USA 87: 5193–5197, 1990
Brown JF, Hanson PJ, Whittle BJR: The nitric oxide donor, S-nitroso-N-acetyl-penicillamine inhibits secretory activity in rat isolated parietal cells. Biochem Biophys Res Commun 195: 1354–1359, 1993
Christophe JP, Frandsen EK, Conlon TP, Krishna G, Gardner JD: Action of cholecystokinin, cholinergic agents, and A-23187 on accumulation of guanosine 3′5′ monophosphate in dispersed guinea pig pancreatic acinar cells. J Biol Chem 251: 4640–4645, 1976
Kapoor CL, Krishna G: A possible role for guanosine 3¢5¢ monophosphate in the stimulus-secretion coupling in exocrine pancreas. Biochim Biophys Acta 544: 102–112, 1978
Gunther GR, Jamieson JD: Increased intracellular cyclic GMP does not correlate with protein discharge from pancreatic acinar cells. Nature 280: 318–320, 1979
Menozzi D, Sato S, Jensen RT, Gardner JD: Cyclic GMP does not inhibit protein kinase C-mediated enzyme secretion in rat pancreatic acini. J Biol Chem 264: 995–999, 1989
Berridge MJ: Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositosides instead of phosphatidylinositol. Biochem J 212: 849–858, 1983
Yule DY, Williams JA: Stimulus-secretion coupling in the pancreatic acinus. In: L.R. Johnson (ed). Physiology of the Gastrointestinal Tract. Raven Press, New York, 1994, pp 1447–1472
Nishizuka Y: The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 3334: 661–665, 1988
Bruzzone R: The molecular basis of enzyme secretion. Gastroenterology 99: 1157–1176, 1990
Ederveen AGH, Van Emst-De Vries SE, De Pont JJHHM, Williems PHGM: Dissimilar effects of the protein kinase C inhibitors, staurosporine and H-7, on cholecystokinin-induced enzyme secretion from rabbit pancreatic acini. Eur J Biochem 193: 291–295, 1990
Francis LP, Camello PJ, Singh J, Salido GM, Madrid JA: Effects of phorbol ester on cholecystokinin octapeptide-evoked exocrine pancreatic secretion in the rat. J Physiol 431: 27–37, 1990
Streb H, Irvine RF, Berridge MJ, Schulz I: Release of calcium from a nonmitochondrial store in pancreatic cells by inositol-1,4,5-trisphosphate. Nature 306: 67–68, 1983
Yule DI, Gallacher DV: Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett 239: 358–362, 1988
Stuenkel EL, Tsunoda Y, Williams JA: Secretagogue-induced calcium mobilization in single pancreatic acinar cells. Biochem Biophys Res Commun 158: 863–869, 1989
Clapham DE: Calcium signalling. Cell 8: 259–268, 1995
Pandol SJ, Schoeffield MS, Fimmel CJ, Muallem S: The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane calcium influx mechanism. J Biol Chem 262: 16963–16968, 1987
Tsunoda Y, Stuenkel EL, Williams JA: Characterization of sustained calcium increase in pancreatic acinar cells and its relation to amylase secretion. Am J Physiol 259: G792–G801, 1990
Pandol S: Pancreatic acinar cell signalling mechanism. Curr Opin Gastroent 10: 485–490, 1994
Putney J: A model for receptor-regulated calcium entry. Cell Calcium 7: 1–12, 1986
Putney JW: Capacitative calcium entry revisited. Cell Calcium 11: 611–624, 1990
Thastrup O, Cullen PJ, Brobak BK, Hanley MR, Dawson AP: Thapsigargin, a tumor promoter, discharges intracellular calcium stores by specific inhibition of the endoplasmic reticulum calcium-ATPase. Biochemistry 8: 2466–2470, 1990
Pandol SJ, Schoeffield-Payne MS: Cyclic GMP mediates the agoniststimulated increase in plasma membrane calcium entry in the pancreatic acinar cell. J Biol Chem 265: 12846–12853, 1990
Xu W, Willis JS: Sodium transport through the amiloride-sensitive sodium-magnesium pathway of hamster red cells. J Membr Biol 141: 277–287, 1994
Gukovskaya AS, Pandol SJ: Dual regulation of cGMP formation by calcium in pancreatic acinar cells. Am J Physiol 268: G900–G907, 1995
Osipchuk YV, Wakui M, Yule DI, Gallacher DV, Petersen OH: Cytoplasmic calcium oscillations evoked by receptor stimulation G-protein activation, internal application of inositol trisphosphate, or calcium: Simultaneous microfluorimetry and calcium-dependent chloride current recording in single pancreatic acinar cells. EMBO J 9: 697–704, 1990
Wakui M, Osipchuk Y, Petersen OH: Receptor-activated cytoplasmic calcium spiking mediated by inositol trisphosphate is due to calciuminduced calcium release. Cell 63: 1025–1032, 1990
Yule DI, Lawrie AM, Gallacher DV: Acetylcholine and cholecystokinin induce different patterns of oscillating calcium signals in pancreatic acinar cells. Cell Calcium 12: 145–151, 1991
Thevenod F, Dehlinger-Kremer M, Kemmer TP, Christian AL, Potter BVL, Schulz I: Characterization of inositol 1,4,5-trisphosphate-sensitive (IsCaP) and insensitive (IisCaP) nonmitochondrial calcium pools in rat pancreatic acinar cells. J Membr Biol 109: 173–186, 1989
Tsunoda Y, Stuenkel EL, Williams JA: Oscillatory model of calcium signalling in rat pancreatic acinar cells. Am J Physiol 258: C147–C155, 1990
Ehrlich BE: Functional properties of intracellular calcium-release channels. Curr Opin Neurobiol 5: 304–309, 1995
Miyazaki S: Inositol trisphosphate receptor-mediated spatiotemporal calcium signalling. Curr Opin Cell Biol 7: 190–196, 1995
Petersen OH: New aspects of cytosolic calcium signalling. News Physiol Sci 11: 13–17, 1996
Lee HC: Potentiation of calcium-induced and caffeine-induced calcium release by cyclic ADP-ribose. J Biol Chem 268: 293–299, 1993
Thorn P, Gerasimenko O, Petersen OH: Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist-evoked cytosolic calcium oscillations in pancreatic acinar cells. EMBO J 13: 2038–2043, 1994
Galione A: Calcium-induced calcium release and its modulation by cyclic ADP-ribose. Trends Pharmacol Sci 13: 304–306, 1992
Vu CQ, Lu P-J, Chen C-S, Jacobson MK: Cyclic ADP-ribose, a calcium mobilising agent derived from NADP. J Biol Chem 271: 4747–4754, 1996
Galione A, White A, Wilmott N, Turner M, Potter BVL, Watson SP: cGMP mobilizes intracellular calcium in sea urchin eggs by stimulating cyclic ADP-ribose system. Nature 365: 456–459, 1993
Francis SH, Corbin JD: Structure and function of cyclic nucleotidedependent protein kinases. Annu Rev Physiol 56: 237–272, 1994
Lei S, Pan Z-H, Aggarwal SK, Chen H-SV, Hartman J, Sucher NJ, Lipton SA: Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron 8: 1992
Lander HM, Sehajpal P, Levine DM, Novogrodsky A: Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol 150: 1509–1516, 1993
Lambert M, Deschodt-Lanckman M, Furnelle J, Christophe J: Similar characteristics of guanine nucleotide regulatory sites involved in adenylate cyclase activation, specific ATPase activity, and cholecystokinin binding in rat pancreatic plasma membranes. J Cycl Nucleotide Res 7: 385–397, 1981
Matozaki T, Williams JA: Regulation of phospholipid hydrolysis in streptolysin-o-permeabilised rat pancreatic acini. Pancreas 7: 59–65, 1992
Neer EJ: Heterotrimeric G proteins: Organisers of transmembrane signals. Cell 80: 249–257, 1995
Lander HM, Sehajpal PK, Novogrodsky A: Nitric oxide signalling: A possible role for G proteins. J Immunol 151: 7182–7187, 1993
Gopalakrishna R, Chen CH, Gundimeda U: Nitric oxide and nitric oxide-generating agents induce a reversible inactivation of protein kinase C activity and phorbol ester binding. J Biol Chem 268: 27180–27185, 1993
Calvo JJ, Garcia-Benito M, Acosta JJ, San Roman JI, Lopez MA, Garcia LJ: Tyrosine phosphorylation of both p125 focal adhesion kinase and paxillin in rat pancreatic acinar cells after nitric oxide stimulation. Digestion 58: 8, 1997
Michalek R, Templeton D: Description of an automated assay for measurement of α-amylase in vitro from rat parotid gland slices. Gen Pharmacol 18: 555–558, 1987
Pearson GT, Singh J, Petersen OH: Adrenergic nervous control of cAMP-mediated amylase secretion in the rat pancreas. Am J Physiol 246: G563–G573, 1984
Wisdom DM, Camello PJ, Salido GM, Singh J: Interaction between secretin and nerve-mediated amylase secretion in the isolated rat pancreas. Exp Physiol 79: 851–863, 1994
Streb H, Schulz I: Regulation of cytosolic free calcium concentration in acinar cells of rat pancreas. Am J Physiol 245: G347–G357, 1983
Francis LP, Lennard R, Singh J: Mechanism of action of magnesium on acetylcholine-evoked secretory responses in isolated rat pancreas. Exp Physiol 75: 669–688, 1990
Juma LMO, Singh J, Adeghate E, Salido GM, Tapia JA: Distribution and effects of nitric oxide on nerve-and acetylcholine-evoked secretory responses in the isolated rat pancreas. J Physiol 504P: 40P, 1997
Ember Z, Yago MD, Singh J: Nitrergic nervous control of amylase secretion and calcium mobilization in the isolated rat pancreas. J Physiol 54P: 24P, 1998
Barnes JM, Barnes NM, Costall B, Horovitz ZP, Naylor RJ: Angiotensin II inhibits the release of [3H]acetylcholine from rat entorhinal cortex in vitro. Brain Res 491: 136–143, 1989
Jennings LJ, Salido GM, Pariente JA, Davison JS, Singh J, Sharkey KA: Control of exocrine secretion in the guinea-pig pancreas by histamine H3 receptors. Can J Physiol Pharmacol 74: 744–752, 1996
Singh J, Pearson GT: Effects of nerve stimulation on enzyme secretion from the in vitro rat pancreas and 3H release after preincubation with catecholamines. Naunyn-Schmiedebergs Arch Pharmacol 327: 228–233, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yago, M.D., Ma~as, M., Ember, Z. et al. Nitric oxide and the pancreas: Morphological base and role in the control of the exocrine pancreatic secretion. Mol Cell Biochem 219, 107–120 (2001). https://doi.org/10.1023/A:1010834611480
Issue Date:
DOI: https://doi.org/10.1023/A:1010834611480